Abstract
Background
There is a need to more fully characterize financial capacity losses in the preclinical and prodromal stages of Alzheimer’s disease (AD) and their pathological substrates.
Objectives
To test the association between financial skills and cortical β-amyloid deposition in aging and subjects at risk for AD.
Design
Cross-sectional analyses of data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI-3) study conducted across 50 plus sites in the US and Canada.
Setting
Multicenter biomarker study.
Participants
243 subjects (144 cognitively normal, 79 mild cognitive impairment [MCI], 20 mild AD).
Measurements
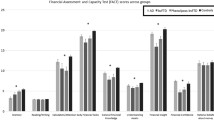
18F-Florbetapir brain PET scans to measure global cortical β-amyloid deposition (SUVr) and the Financial Capacity Instrument Short Form (FCI-SF) to evaluate an individual’s financial skills in monetary calculation, financial concepts, checkbook/register usage, and bank statement usage. There are five sub scores and a total score (range of 0–74) with higher scores indicating better financial skill.
Results
FCI-SF total score was significantly worse in MCI [Cohen’s d= 0.9 (95%CI: 0.6–1.2)] and AD subjects [Cohen’s d=3.1(CI: 2.5–3.7)] compared to normals. Domain scores and completion times also showed significant difference. Across all subjects, higher cortical β-amyloid SUVr was significantly associated with worse FCI-SF total score after co-varying for age, education, and cognitive score [Cohen’s f2=0.751(CI: 0.5–1.1)]. In cognitively normal subjects, after covarying for age, gender, and education, higher β -amyloid PET SUVr was associated with longer task completion time [Cohen’s f2=0.198(CI: 0.06–0.37)].
Conclusion
Using a multicenter study sample, we document that financial capacity is impaired in the prodromal and mild stages of AD and that such impairments are, in part, associated with the extent of cortical β-amyloid deposition. In normal aging, β-amyloid deposition is associated with slowing of financial tasks. These data confirm and extend prior research highlighting the utility of financial capacity assessments in at risk samples.





Similar content being viewed by others
References
Marson DC, Sabatino SP. Financial capacity in an aging society. Generations. 2012:36(2):6–11
Sherod MG, Griffith HR, Copeland J, et al. Neurocognitive predictors of financial capacity across the dementia spectrum: normal aging, MCI, and Alzheimer’s disease. J International Neuropsychological Society. 2009;15(2):258–267.
Marson D. Investigating Functional Impairment in Preclinical Alzheimer’s Disease. J Prev Alzheimers Dis. 2015;2(1):4–6.
Griffith HR, Belue K, Sicola A, et al. Impaired financial abilities in mild cognitive impairment: a direct assessment approach. Neurology. 2003;60:449–57.
Triebel KL, Martin R, Griffith R, et al., Declining financial capacity in mild cognitive impairment. Neurology. 2009:73(12):928–934.
Gerstenecker A, Triebel KL, Martin R, et al. Both financial and cognitive changes predict clinical progression in MCI. Alzheimer’s Disease and Associated Disorders. 2016:30(1):27–34
Howe N. The Graying of Wealth. Hedgeye. 2017. https://app.hedgeye.com/insights/62871-the-graying-of-wealth. Accessed: 07 Feb 2019.
Bricker J, Dettling, LJ, Henriques, A, et al. Changes in U.S. Family Finances from 2013 to 2016: Evidence from the Survey of Consumer Finances. Federal Reserve Bulletin. 2017:103(4)
Laumann EO, Leitsch SA, Waite LJ. Elder mistreatment in the United States: prevalence estimates from a nationally representative study. J Gerontol B Psychol Sci Soc Sci. 2008;63(4):S248–S254.
Marson D and Zebley L. The Other Side of the Retirement Years: Cognitive Decline, Dementia, and Loss of Financial Capacity. J. Retire. Plan. 2001:4:30–39
Ghesquiere AR, McAfee C, and Burnett J. Measures of Financial Capacity: A Review. The Gerontologist, 2017: 76–95.
Marson DC, Sawrie SM, Snyder S, et al. Assessing Financial Capacity in Patients With Alzheimer Disease: A Conceptual Model and Prototype Instrument. Arch Neurol. 2000;57(6):877–884.
Martin RC, Gerstenecker A, Triebel KL, et al. Declining Financial Capacity in Mild Cognitive Impairment: A Six-Year Longitudinal Study. Archives of Clinical Neuropsychology. 2018
Griffith HR, Stewart CC, Stoeckel LE, et al. Magnetic resonance imaging volume of the angular gyri predicts financial skill deficits in people with amnestic mild cognitive impairment. J Am Geriatr Soc. 2010;58(2):265–74.
Stoeckel LE, Stewart CC, Griffith HR, et al. MRI volume of the medial frontal cortex predicts financial capacity in patients with mild Alzheimer’s disease. Brain Imaging Behav. 2013;7(3):282–92.
Marson DC, Triebel KL, Gerstenecker A, Martin RC, Edwards K, Pankratz VS, McPherson T, Swenson-Dravis D, Petersen RC. Detecting functional impairment in preclinical Alzheimer’s disease using a brief performance measure of financial skills (in preparation)
Marson DC, Triebel KL, Gerstenecker A, Martin RC, Edwards K, Pankratz VS, Swenson-Dravis D, Petersen RC. Detecting declining financial skills in preclinical Alzheimer’s disease: the Financial Capacity Instrument-Short Form; Poster presentation at the 10th annual conference of the International Society for CNS Clinical Trials and Methodology (ISCTM); Boston, Massachusettts. Oct 7, 2014.
Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011:305(3):275–83.
Doraiswamy PM, Sperlin RA, Johnson K, et al. Florbetapir F 18 amyloid PET and 36-month cognitive decline: a prospective multicenter study. Molecular psychiatry. 2014:19(9)1044–51.
Chételat G, La Joie R, Villain N, et al. Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. NeuroImage: Clinical. 2013:2:356–65.
Veitch DP, Weiner MW, Aisen PS, et al. Understanding disease progression and improving Alzheimer’s disease clinical trials: Recent highlights from the Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. 2019:15(1):106–152
Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimer’s & Dementia, 2005:1(1):55–66.
Weiner MW, Veitch DP, Aisen PS, et al. The Alzheimer’s Disease Neuroimaging Initiative 3: continued innovation for clinical trial improvement. Alzheimer’s & Dementia. 2017:13(5):561–571.
Weiner M. Alzheimer’s Disease Neuroimaging Initiative 3 (ADNI3) Protocol. 2016. https://clinicaltrials.gov/ct2/show/NCT02854033. Accessed: 27 Dec 2018.
Landau S. and Jagust W. Florbetapir processing methods. 2011
Gerstenecker A, Hoagey DA, Marson DC, et al. White Matter Degradation is Associated with Reduced Financial Capacity in Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimers Dis. 2017:60(2):537–547
Prescott JW, Guidon A, Doraiswamy PM, et al. The Alzheimer structural connectome: changes in cortical network topology with increased amyloid plaque burden. Radiology. 2014;273(1):175–84.
Jacobs HIL, Hedden T, Schultz AP, et al. Structural tract alterations predict downstream tau accumulation in amyloid-positive older individuals. Nat Neurosci. 2018;21(3):424–431.
Gerstenecker A, Eakin A, Triebel K, et al. Age and education corrected older adult normative data for a short form version of the Financial Capacity Instrument. Psychological assessment. 2016:28(6):737.
Acknowledgments
We are grateful to Dr. Daniel Marson for his valuable insights. Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.ucla.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wpcontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Funding
Funding: Funding for data collection were funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904, Michael Weiner, PI) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (http://www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing Financial Interests: MWW is the principal investigator for ADNI and all other authors are ADNI investigators at Duke. PMD is supported by NIH, DOD and Cure Alzheimer’s Fund, has served as an advisor to and/or received grants from several companies and non-profits in this field, and owns stock in or serves on boards of companies whose products are not discussed here. Other co-authors may also have received grants or advisory fees from companies for other projects.
Ethical standards: The institutional review board at Duke University Health System and at each ADNI site reviewed and approved the ADNI protocol. All subjects and their legal representatives, where appropriate, gave written informed consent prior to data collection.
Conflict of interest: We reported this under the existing para titled Competing Financial Interests.
Rights and permissions
About this article
Cite this article
Tolbert, S., Liu, Y., Hellegers, C. et al. Financial Management Skills in Aging, MCI and Dementia: Cross Sectional Relationship to 18F-Florbetapir PET Cortical β-amyloid Deposition. J Prev Alzheimers Dis 6, 274–282 (2019). https://doi.org/10.14283/jpad.2019.26
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jpad.2019.26