The Out-of-Pocket Cost Burden for Specialty Drugs in Medicare Part D in 2019
Medicare Part D enrollees without low income subsidies can expect to pay thousands of dollars out of pocket for a single specialty tier drug in 2019. For many specialty tier drugs, the majority of these costs will occur in the catastrophic phase of the benefit.
Part D enrollees can face thousands of dollars in annual out-of-pocket costs if they take expensive drugs, despite having catastrophic coverage. Expected annual out-of-pocket costs in 2019 average $8,109 across the 28 specialty tier drugs covered by some or all plans in this analysis. For 28 of the 30 studied specialty drugs used to treat four health conditions—cancer, hepatitis C, multiple sclerosis (MS), and rheumatoid arthritis (RA)—expected annual out-of-pocket costs for a single drug in 2019 range from $2,622 for Zepatier, a treatment for hepatitis C, to $16,551 for Idhifa, a leukemia drug. Two of the 30 drugs are not covered by any plan in our analysis. (See Tables 1 and 2 for drug-specific cost and coverage information.)
Part D enrollees taking high-cost specialty tier drugs often incur significant costs in the catastrophic coverage phase of the benefit because the catastrophic threshold is not an absolute limit on out-of-pocket spending. For the 28 specialty tier drugs in our analysis covered by some or all plans, the share of annual out-of-pocket costs that would be incurred in the catastrophic phase in 2019 ranges from 13 percent for Zepatier to 86 percent for Idhifa; for 19 of these drugs, enrollees can expect to pay more than half of their annual out-of-pocket cost in the catastrophic phase. On average across these 28 specialty drugs, 61 percent of annual out-of-pocket costs occur above the catastrophic threshold in 2019, which translates to $5,444 in out-of-pocket costs in the catastrophic phase alone.
out-of-pocket Costs for SELECtED specialty tier drugs in 2019, by Condition
Cancer
Medicare Part D enrollees without low-income subsidies who take any of the specialty tier drugs in our analysis for various types of cancer would pay more out of pocket for these medications in 2019 than enrollees who take any of the specialty drugs for the other health conditions in this analysis. Fourteen of the 15 studied specialty tier cancer drugs are covered by all plans, and the median annual out-of-pocket cost for each of these drugs exceeds $8,000. One of the 15 cancer drugs, Gleevec, is not covered by any plan in our analysis in 2019, but the generic equivalent, imatinib mesylate, is covered by all plans, which is sufficient to meet the formulary coverage requirement that plans cover all or substantially all drugs in six so-called “protected” classes, including cancer drugs.
Expected annual out-of-pocket costs in 2019 for the 14 covered specialty tier cancer drugs range from $8,181 for Zytiga (for prostate cancer) to $16,551 for Idhifa (for leukemia) (Figure 2). Part D enrollees taking any of the brand-name cancer drugs in our analysis for the full year would pay at least 70 percent of their total annual out-of-pocket costs above the catastrophic threshold in 2019. For example, 84 percent of an enrollee’s out-of-pocket costs for Revlimid, a drug to treat multiple myeloma, would occur in the catastrophic phase, which translates to $12,186 in costs for this drug in the catastrophic phase alone in 2019. For imatinib mesylate, the generic equivalent for Gleevec, the share of costs above the catastrophic coverage threshold is lower (43%) because enrollees taking this drug do not receive a manufacturer discount in the coverage gap and therefore would incur higher out-of-pocket costs below the catastrophic coverage threshold.
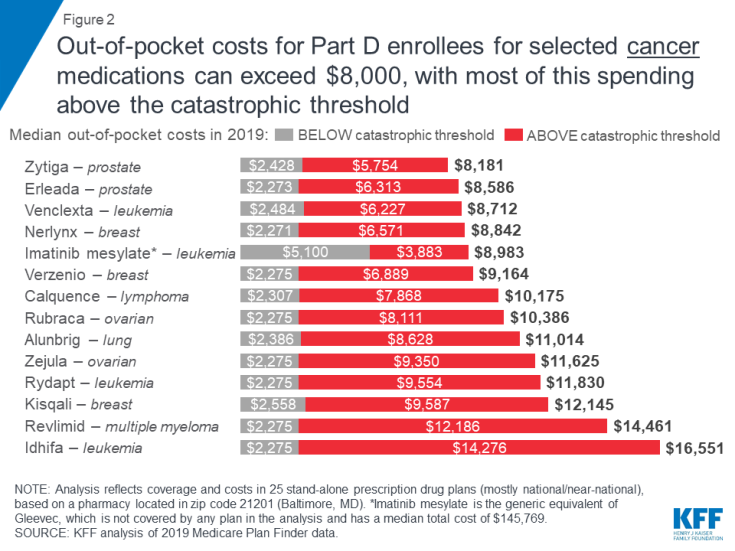
Figure 2: Out-of-pocket costs for Part D enrollees for selected cancer medications can exceed $8,000, with most of this spending above the catastrophic threshold
Hepatitis C
Out-of-pocket spending for breakthrough therapies used to treat and cure hepatitis C represents a significant burden for Part D enrollees who do not receive low-income subsidies, even though costs for some of these drugs have fallen over time as new competitor products have come to market.
Expected annual out-of-pocket costs in 2019 for six of the seven hepatitis C drugs in our analysis range from $2,622 for Zepatier to $6,338 for Harvoni (Figure 3). (A previously published version of this brief reported lower costs for Harvoni, based on the default quantity in the Medicare Plan Finder (28 pills/year), which is lower than the recommended quantity (84 pills/year). The estimates in this report have been updated to reflect the recommended quantity, resulting in higher costs for Harvoni. See Methods for additional details.) One of the seven drugs, Viekira Pak, is not covered by any of the plans in our analysis. For four hepatitis C drugs in our analysis (Epclusa, Vosevi, Sovaldi, and Harvoni), Part D enrollees can expect to pay more than half of their total out-of-pocket costs in the catastrophic phase in 2019, based on a full year of use. For example, 60 percent of an enrollee’s out-of-pocket costs for Sovaldi would occur in the catastrophic phase, which translates to $3,368 in costs for this drug in the catastrophic phase alone in 2019.
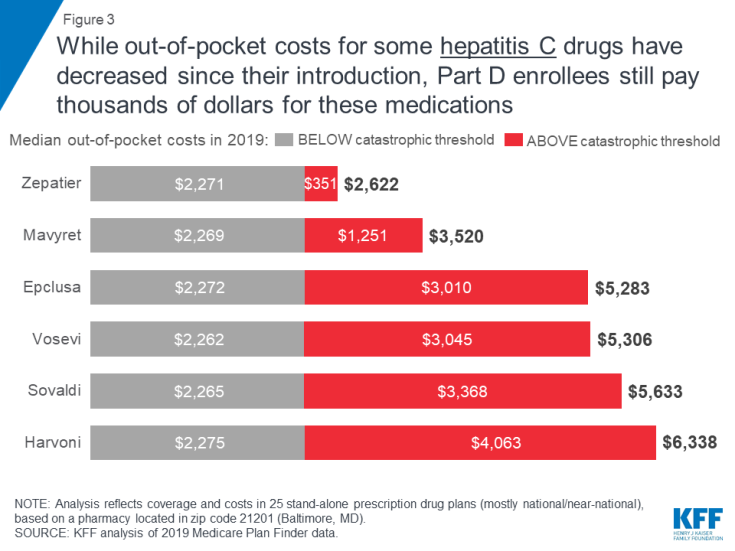
Figure 3: While out-of-pocket costs for some hepatitis C drugs have decreased since their introduction, Part D enrollees still pay thousands of dollars for these medications
Multiple Sclerosis
Expected annual out-of-pocket costs in 2019 for the four specialty tier drugs in our analysis used to treat multiple sclerosis range from $6,507 for Avonex to $7,409 for glatiramer acetate (the generic equivalent of Copaxone) (Figure 4). An enrollee taking any of the three brand-name MS drugs in our analysis (Avonex, Copaxone, and Tecfidera) can expect to pay more than half of their total annual out-of-pocket costs in the catastrophic phase.
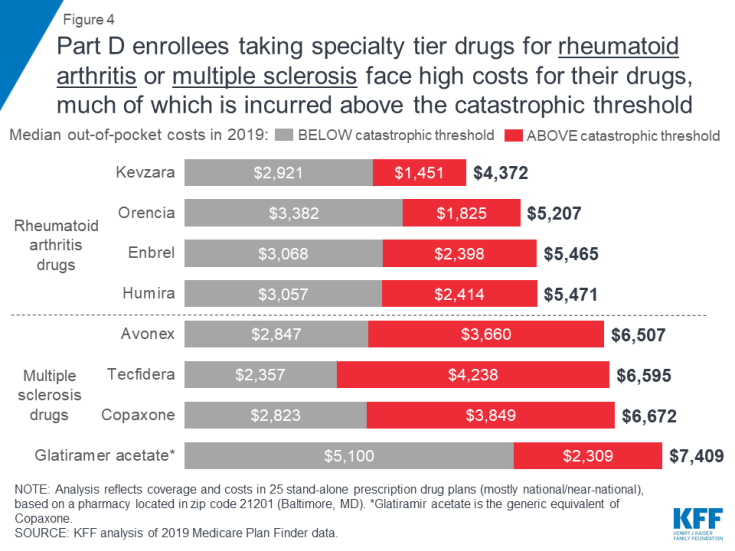
Figure 4: Part D enrollees taking specialty tier drugs for rheumatoid arthritis or multiple sclerosis face high costs for their drugs, much of which is incurred above the catastrophic threshold
Expected annual out-of-pocket costs for glatiramer acetate are actually higher than costs for the brand Copaxone in 2019—and higher than out-of-pocket costs for the other branded MS drugs—while the share of out-of-pocket costs above the catastrophic threshold is lower. This is because enrollees who take the brand Copaxone would reach the catastrophic phase sooner than those who take the generic equivalent, because they receive a 70 percent manufacturer discount on the brand, which counts towards the annual out-of-pocket spending amount that triggers catastrophic coverage. This discount is not offered to those who use generic drugs, so more of their annual spending would occur below the catastrophic coverage threshold, where they face a higher coinsurance rate in the coverage gap (37%) than those who take brands (25%).
Rheumatoid Arthritis
For Part D enrollees taking any of the four specialty tier drugs in our analysis for rheumatoid arthritis, expected annual out-of-pocket costs in 2019 range from $4,372 for Kevzara to $5,471 for Humira (Figure 4). At least one-third of total annual out-of-pocket costs for each of these drugs would be incurred by Part D enrollees after their spending exceeds the catastrophic threshold. For example, Part D enrollees taking either Humira or Enbrel would pay 44 percent of their total annual out-of-pocket costs above the catastrophic threshold in 2019, based on a full year of use, which translates to costs of $2,414 for Humira and $2,398 for Enbrel in the catastrophic phase alone.
Medicare Part D coverage of specialty tier drugs varies across plans, which has implications for enrollees’ access and costs.
Part D plans must adhere to specific formulary guidelines laid out by Medicare, such as covering a minimum of two drugs in each therapeutic class and covering all or substantially all drugs in six protected classes, but plans can omit drugs from their formularies for a variety of reasons. Having flexibility in formulary design means that plan sponsors may be able to leverage steeper rebates or discounts from a drug manufacturer by excluding a competitor’s product from its formulary or placing the competitor’s drug on a non-preferred tier. Part D enrollees may benefit from negotiated rebates if lower overall plan costs translates to lower plan premiums. But Part D enrollees who are prescribed a drug that is not covered by their plan would either have to pay the total cost of the drug on their own (which is unlikely in the case of expensive specialty tier medications), switch to an alternative medication that is covered by their plan, or appeal to their plan to cover their drug that is not on the formulary.
Sixteen of the 30 studied specialty drugs are covered by all plans in our analysis in 2019, 14 of which are for cancer, which is one of the six protected classes. In contrast, 12 of the studied specialty drugs are not covered by some plans and two drugs are not covered by any plan in our analysis (Figure 5).
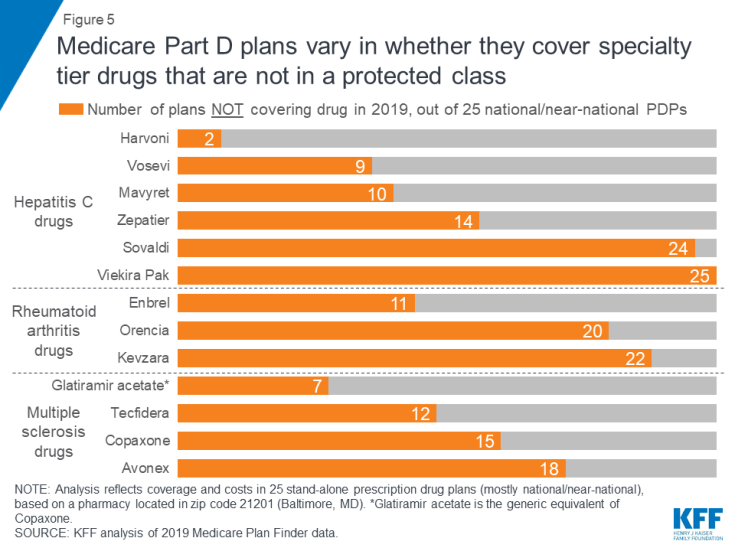
Figure 5: Medicare Part D plans vary in whether they cover specialty tier drugs that are not in a protected class
Some plans cover a larger number of specialty drugs to treat each condition than other plans (outside of the protected-class cancer drugs). None of the 25 plans in this analysis covers all of the specialty tier drugs for hepatitis C, multiple sclerosis, or rheumatoid arthritis, and there is wide variation across plans in how many drugs they cover for each condition. For example, of the four MS drugs in our analysis, nine of the 25 plans cover just one of the four drugs, another nine plans cover two, and four plans cover three.
expected Annual Costs for SELECTED Specialty tier drugs When noT covered
The annual cost for a specialty tier drug that is not covered on a plan’s formulary is substantially greater than the cost of that drug when it is covered. For the 14 specialty drugs in our analysis that are not covered by some or all plans, the median total annual cost when off formulary ranges from $26,209 for Zepatier to $145,769 for Gleevec—amounts that exceed the limits of affordability for the vast majority of Part D enrollees.
For the 12 specialty tier drugs in our analysis covered by some but not all plans, the median annual cost among plans that do not cover the drug is at least 10 times higher than the median out-of-pocket cost when it is covered (Figure 6). For example, the total median annual off-formulary cost for the hepatitis C drug Mavyret, is $47,521 compared to $3,520 in median annual out-of-pocket costs when covered; the total median annual off-formulary cost for the MS drug Tecfidera is $106,070, compared to $6,595 in median out-of-pocket costs when covered.
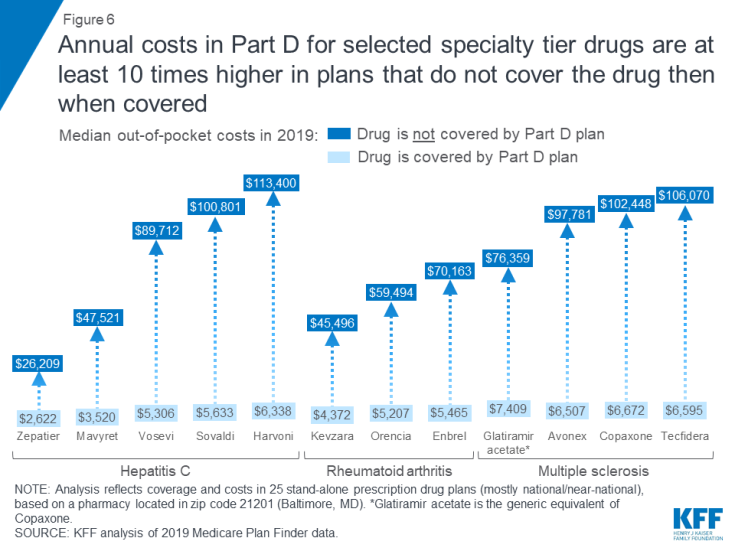
Figure 6: Annual costs in Part D for selected specialty tier drugs are at least 10 times higher in plans that do not cover the drug then when covered
Between 2016 and 2019, the median annual out-of-pocket cost for several specialty tier drugs in our analysis increased by 12 percent, on average, even as the coverage gap for brands was closing.
To examine the change in out-of-pocket costs for specialty tier drugs over time, we compared 2016 and 2019 costs for 10 of the 30 drugs in our current analysis that were included in our prior study and covered by at least one plan in 2019. In 2019, expected annual out-of-pocket costs for eight of the 10 specialty tier drugs are 12 percent higher than in 2016, on average (an increase of $873) (Figure 7, Table 3). For two of the 10 drugs, Harvoni and Sovaldi—both used to treat hepatitis C—median annual out-of-pocket costs in 2019 are 13 percent lower than in 2016, on average (a decrease of $896), possibly due to the entrance of competitor products since the end of 2015, and other factors related to changes in the benefit design (lower cost sharing in the gap, the manufacturer price discount, and a higher out-of-pocket threshold for catastrophic coverage), combined with the limited duration of treatment for hepatitis C drugs, that translate to a reduction in annual out-of-pocket costs for enrollees taking these two drugs in 2019. Of the 12 specialty tier drugs in our previous analysis, two drugs (Viekira Pak and Gleevec) are not covered by any plan in our analysis in 2019, so we could not calculate a change in the median out-of-pocket cost for these drugs.
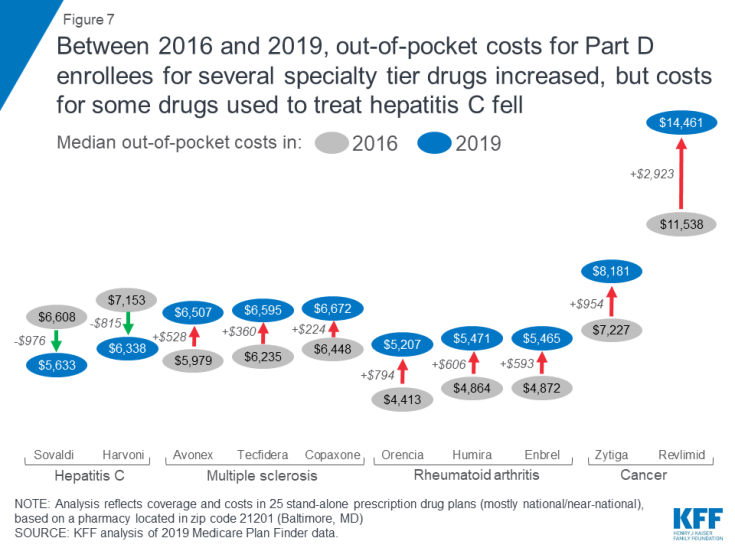
Figure 7: Between 2016 and 2019, out-of-pocket costs for Part D enrollees for several specialty tier drugs increased, but costs for some drugs used to treat hepatitis C fell
For the eight specialty drugs in our analysis in both years where Part D enrollees can expect to pay more out-of-pocket in 2019 than in 2016, median annual cost increases range from $224 for the MS drug Copaxone to $2,923 for Revlimid, a drug for multiple myeloma. The expected annual out-of-pocket cost for Copaxone is $6,672 in 2019, up from $6,448 in 2016, while the expected annual cost for Revlimid increased from $11,538 to $14,461 over these years. Conversely, median annual out-of-pocket costs for two hepatitis C specialty drugs decreased between 2016 and 2019—by $976 for Sovaldi (from $6,608 to $5,633) and $815 for Harvoni (from $7,153 to $6,338).
With the now-complete closure of the Part D coverage gap for brand-name drugs, enrollees who take selected specialty tier drugs can expect to face lower out-of-pocket costs below the catastrophic threshold in 2019 than in 2016, but higher costs above. Under the current benefit design, beneficiaries pay 25 percent of total costs for brand-name drugs in the coverage gap in 2019, down from 45 percent in 2016, and the manufacturer discount on brand-name drugs in the coverage gap increased from 50 percent to 70 percent between 2018 and 2019, generating even greater savings for enrollees taking brand-name drugs with spending in the coverage gap.
As an example, for Humira, median out-of-pocket costs below the catastrophic coverage threshold decreased by $99 between 2016 and 2019 (from $3,155 to $3,057), while costs above the catastrophic threshold increased by $705 over these years (from $1,709 to $2,414)—and in total, expected annual out-of-pocket costs for Humira are $606 (12%) higher in 2019 than in 2016 (Figure 8).
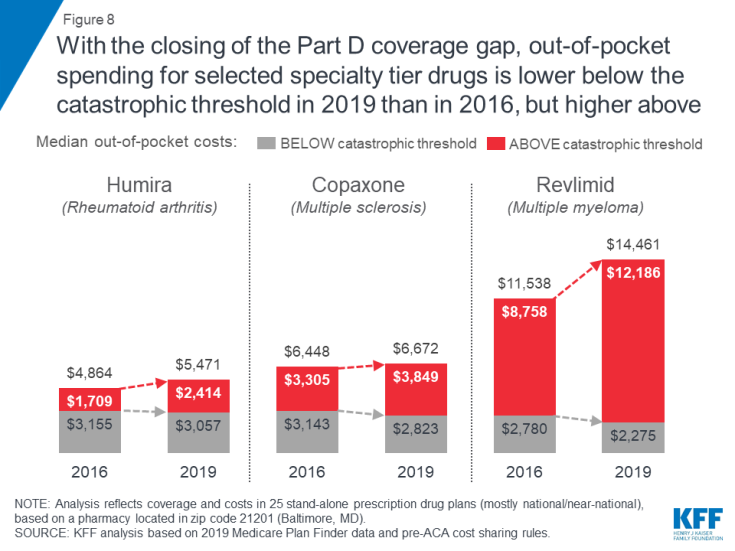
Figure 8: With the closing of the Part D coverage gap, out-of-pocket spending for selected specialty tier drugs is lower below the catastrophic threshold in 2019 than in 2016, but higher above
Thus, while the closing of the coverage gap has reduced out-of-pocket costs for Part D enrollees who reach the gap but not the catastrophic phase of the benefit, enrollees can expect to see higher annual out-of-pocket costs for several specialty tier drugs in 2019 compared to 2016. This is because higher underlying prices for these drugs translate to higher out-of-pocket costs in the catastrophic phase of the benefit, where beneficiaries pay 5 percent of the price of the drug, which more than offsets any savings from lower costs in the gap.
Discussion
This analysis shows that Medicare Part D enrollees who do not receive low-income subsidies can expect to pay thousands of dollars in out-of-pocket costs for a single specialty tier drug in 2019, even though the Part D coverage gap for brands is now fully closed. Although Part D offers catastrophic coverage for high drug costs, beneficiaries can still face substantial out-of-pocket costs for expensive medications, including many drugs for cancer, hepatitis C, multiple sclerosis, and rheumatoid arthritis, because there is no hard cap on spending in the Part D benefit. Part D enrollees who need specialty tier drugs that are not covered by their plan could be exposed to substantial costs—which would likely mean not filling a prescription for the off-formulary drug and instead taking a therapeutic substitute.
The high cost of prescription drugs has contributed to growing public support for the government to take action to address drug costs. A majority (80%) of Americans say the cost of prescription drugs is unreasonable, and there is broad support among Democrats, Republicans, and Independents for several different options to lower drug costs. The Trump Administration has endorsed a number of proposals to address rising drug costs for Medicare and beneficiaries, including adding a hard cap on Part D out-of-pocket spending. The Administration has also proposed changes to reduce spending on drugs covered under Part B, including a proposal to benchmark U.S. prices against prices set internationally. Lawmakers in Congress are also moving forward with proposals to reduce drug costs, including allowing Medicare to negotiate drug costs, letting patients import prescription drugs from Canada, and expediting the introduction of generic and other prescription drugs. As more expensive drugs come to market in the future, the high cost of prescription drugs will continue to be a pressing issue for policymakers and a major pocketbook issue for patients.
