Protective Role of Phenolic Compounds from Whole Cardamom (Elettaria cardamomum (L.) Maton) against LPS-Induced Inflammation in Colon and Macrophage Cells
Abstract
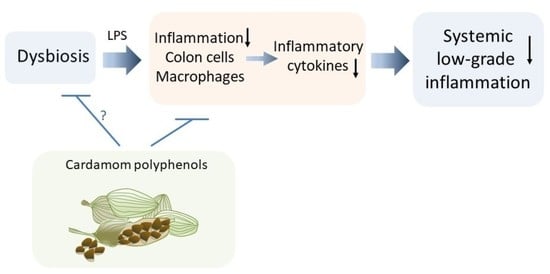
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cardamom Samples
2.3. Sample Preparation for Polyphenol Analysis and LC-MS Profiling
2.4. Sample Preparation for Terpenoid Analysis and GC-MS Profiling
2.5. Cell Culture for Inflammation Studies
2.6. Cell Viability Test
2.7. Detection of Extracellular Nitric Oxide and Intracellular Reactive Oxygen Species Production
2.8. Total RNA and Gene Expression Analysis (Real-Time qRT-PCR)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Polyphenol Profiles from Whole Cardamom, Skin, and Seeds by LC-MS
3.2. Volatile Chemistry of Essential Oils from Whole Cardamom, Skin, and Seeds by GC-MS
3.3. Colon and Macrophage Cell Viability, Reactive Oxygen Species (ROS), and Nitric Oxide (NO) Production
3.4. Effects in Pro-Inflammatory Genes in Colon Cells
3.5. Effects in Pro-Inflammatory Genes in Macrophages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| NO | Nitric oxide |
| LPS | Lipopolysaccharide |
| NFkβ | Nuclear factor kappa β |
| TNFα | Tumor necrosis factor alpha |
| IL-6 | Interleukin 6 |
| COX2 | Cyclooxygenase 2 |
| LXRα | Liver x receptor alpha |
| RNA | ribonucleic acid |
| PCR | Polymerase chain reaction |
| PPARγ | Peroxisome proliferator-activated receptor gamma |
| DMSO | Dimethyl sulfoxide |
| NOX | NADPH oxidase (nicotinamide adenine dinucleotide phosphate oxidase) |
| iNOS | Inducible nitric oxide synthase |
References
- USDA-FAS. Cardamom—The 3Gs—Green Gold of Guatemala. Global Agricultural Information Network; GT-1404; USDA-FAS: Washington, DC, USA, 2014. [Google Scholar]
- Cisneros-Zevallos, L. The power of plants: How fruit and vegetables work as source of nutraceuticals and supplements. Int. J. Food Sci. Nutr. 2021, 72, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Arpitha, S.; Srinivasan, K.; Sowbhagya, H.B. Anti-inflammatory effect of resin fraction of cardamom (Elettaria cardamomum) in carrageenan-induced rat paw edema. PharmaNutrition 2019, 10, 100165. [Google Scholar] [CrossRef]
- Han, X.; Parker, T.L. Cardamom (Elettaria cardamomum) essential oil significantly inhibits vascular cell adhesion molecule 1 and impacts genome-wide gene expression in human dermal fibroblasts. Cogent. Med. 2017, 4, 1308066. [Google Scholar] [CrossRef]
- Souissi, M.; Azelmat, J.; Chaieb, K.; Grenier, D. Antibacterial and anti-inflammatory activities of cardamom (Elettaria cardamomum) extracts: Potential therapeutic benefits for periodontal infections. Anaerobe 2020, 61, 102089. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas Garza, G.R.; Elizondo Luévano, J.H.; Bazaldúa Rodríguez, A.F.; Chávez Montes, A.; Pérez Hernández, R.A.; Martínez Delgado, A.J.; López Villarreal, S.M.; Rodríguez Rodríguez, J.; Sánchez Casas, R.M.; Castillo Velázquez, U.; et al. Benefits of Cardamom (Elettaria cardamomum (L.) Maton) and Turmeric (Curcuma longa L.) Extracts for Their Applications as Natural Anti-Inflammatory Adjuvants. Plants 2021, 10, 1908. [Google Scholar] [CrossRef] [PubMed]
- Mehjabeen, M.A.; Noorjahan, F.-S.; Rehman, A.B. The Role of Elettaria cardamomum (L.) Maton in Inflammatory, Gastrointestinal and Stress Disorders. Int. J. Pharm. Phytopharmacol. Res. 2015, 4, 302–305. [Google Scholar]
- Farivar, M.; Hooshmandi, Z.; Setorki, M.; Amini, S. Effect of Elettaria cardamomum essential oil and its main component (1, 8-cineole) on brain damage caused by cerebral hypoperfusion by suppressing apoptosis and inflammation in rats. Iranian Red. Crescent Med. J. 2020, 22. [Google Scholar] [CrossRef]
- Cheshmeh, S.; Ghayyem, M.; Khamooshi, F.; Heidarzadeh-Esfahani, N.; Rahmani, N.; Hojati, N.; Mosaieby, E.; Moradi, S.; Pasdar, Y. Green cardamom plus low-calorie diet can decrease the expression of inflammatory genes among obese women with polycystic ovary syndrome: A double-blind randomized clinical trial. Eat. Weight. Disord. 2020, 27, 821–830. [Google Scholar] [CrossRef]
- Kazemi, S.; Yaghooblou, F.; Siassi, F.; Rahimi Foroushani, A.; Ghavipour, M.; Koohdani, F.; Sotoudeh, G. Cardamom supplementation improves inflammatory and oxidative stress biomarkers in hyperlipidemic, overweight, and obese pre-diabetic women: A randomized double-blind clinical trial. J. Sci. Food Agric. 2017, 97, 5296–5301. [Google Scholar] [CrossRef]
- Alkhalifah, E.A.R.; Alobaid, A.A.; Almajed, M.A.; Alomair, M.K.; Alabduladheem, L.S.; Al-Subaie, S.F.; Akbar, A.; Attimarad, M.V.; Younis, N.S.; Mohamed, M.E. Cardamom Extract Alleviates the Oxidative Stress, Inflammation and Apoptosis Induced during Acetaminophen-Induced Hepatic Toxicity via Modulating Nrf2/HO-1/NQO-1 Pathway. Curr. Issues Mol. Biol. 2022, 44, 5390–5404. [Google Scholar] [CrossRef]
- Al-Yousef, H.M.; Alqahtani, A.S.; Hassan, W.H.B.; Alzoubi, A.; Abdelaziz, S. Chemical Profile, In Vitro Antioxidant, Pancreatic Lipase, and Alpha-Amylase Inhibition Assays of the Aqueous Extract of Elettaria cardamomum L. Fruits. J. Chem. 2021, 2021, 5583001. [Google Scholar] [CrossRef]
- Gilani, A.H.; Jabeen, Q.; Khan, A.U.; Shah, A.J. Gut modulatory, blood pressure lowering, diuretic and sedative activities of cardamom. J. Ethnopharm. 2008, 115, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Jain, V.; Katewa, S.S. Blood pressure lowering, fibrinolysis enhancing and antioxidant activities of cardamom (Elettaria cardamomum). Indian. J. Biochem. Biophys. 2009, 46, 503–506. [Google Scholar] [PubMed]
- Rahman, M.M.; Alam, M.N.; Ulla, A.; Sumi, F.A.; Subhan, N.; Khan, T.; Alam, M.A. Cardamom powder supplementation prevents obesity, improves glucose intolerance, inflammation and oxidative stress in liver of high carbohydrate high fat diet induced obese rats. Lipids Health Disease 2017, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Nitasha Bhat, G.M.; Nayak, N.; Vinodraj, K.; Chandralekha, N.; Mathai, P.; Cherian, J. Comparison of the efficacy of cardamom (Elettaria cardamomum) with pioglitazone on dexamethasone-induced hepatic steatosis, dyslipidemia, and hyperglycemia in albino rats. J. Adv. Pharma Tech. Res. 2015, 6, 136–140. [Google Scholar] [CrossRef]
- Kawada, T.; Goto, T.; Hirai, S.; Yu, R.; Takahashi, N. Obesity and nuclear receptors: Effective genomic strategies in functional foods. In Nutrigenomics and Proteomics in Health and Disease; Mine, Y., Miyashita, K., Shahidi, F., Eds.; Willey-Blakwell: Hoboken, NJ, USA, 2009; pp. 47–58. [Google Scholar]
- Zhu, W.; Lönnblom, E.; Förster, M.; Johannesson, M.; Tao, P.; Meng, L.; Lu, S.; Holmdahl, R. Natural polymorphism of Ym1 regulates pneumonitis through alternative activation of macrophages. Sci. Adv. 2020, 6, eaba9337. [Google Scholar] [CrossRef]
- Verstockt, B.; Verstockt, S.; Veny, M.; Dehairs, J.; Arnauts, K.; Van Assche, G.; De Hertogh, G.; Vermeire, S.; Salas, A.; Ferrante, M. Expression Levels of 4 Genes in Colon Tissue Might Be Used to Predict Which Patients Will Enter Endoscopic Remission After Vedolizumab Therapy for Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2020, 18, 1142–1151.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittgen, T.D. Analyzing real-time PCR data by the comparative C-T method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef] [Green Version]
- Tian, R.-R.; Pan, Q.-H.; Zhan, J.-C.; Li, J.-M.; Wan, S.-B.; Zhang, Q.-H.; Huang, W.-D. Comparison of phenolic acids and flavan-3-ols during wine fermentation of grapes with different harvest times. Molecules 2009, 14, 827–838. [Google Scholar] [CrossRef] [Green Version]
- Strupat, K.; Karas, M.; Hillenkamp, F. 2, 5-Dihydroxybenzoic acid: A new matrix for laser desorption—Ionization mass spectrometry. Intl. J. Mass. Spectrom. Ion Proc. 1991, 111, 89–102. [Google Scholar] [CrossRef]
- Crumpton, J.B.; Zhang, W.; Santos, W.L. Facile analysis and sequencing of linear and branched peptide boronic acids by MALDI mass spectrometry. Anall. Chem. 2011, 83, 3548–3554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, N.R.; Karthikeyan, A.; Karthikeyan, S.; Reddy, B.V. Inhibitory effect of caffeic acid on cancer cell proliferation by oxidative mechanism in human HT-1080 fibrosarcoma cell line. Mol. Cell. Biochem. 2011, 349, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.). J. Agric Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, M.C.; Barroso, C.G.; Pérez-Bustamante, J.A. Analysis of polyphenolic compounds of different vinegar samples. Eur. Food Res. Technol. 1994, 199, 29–31. [Google Scholar]
- Kobayashi, S.; Uyama, H.; Kimura, S. Enzymatic polymerization. Chem. Rev. 2001, 101, 3793–3818. [Google Scholar] [CrossRef]
- Sakai, S.; Kawamata, H.; Kogure, T.; Mantani, N.; Terasawa, K.; Umatake, M.; Ochiai, H. Inhibitory effect of ferulic acid and isoferulic acid on the production of macrophage inflammatory protein-2 in response to respiratory syncytial virus infection in RAW264. 7 cells. Mediat. Inflamm. 1999, 8, 173–175. [Google Scholar] [CrossRef]
- Valentao, P.; Fernandes, E.; Carvalho, F.; Andrade, P.; Seabra, R.; Bastos, M. Antioxidant activity of Centaurium erythraea infusion evidenced by its superoxide radical scavenging and xanthine oxidase inhibitory activity. J. Agric. Food Chem. 2001, 49, 3476–3479. [Google Scholar] [CrossRef]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Identifying peach and plum polyphenols with chemopreventive potential against estrogen-independent breast cancer cells. J. Agric. Food Chem. 2009, 57, 5219–5226. [Google Scholar] [CrossRef]
- Stacewicz-Sapuntzakis, M.; Bowen, P.E.; Hussain, E.A.; Damayanti-Wood, B.I.; Farnsworth, N.R. Chemical composition and potential health effects of prunes: A functional food? Crit. Rev. Food Sci. Nutr. 2001, 41, 251–286. [Google Scholar] [CrossRef] [PubMed]
- Cynkar, W.; Cozzolino, D.; Dambergs, B.; Janik, L.; Gishen, M. Feasibility study on the use of a head space mass spectrometry electronic nose (MS e_nose) to monitor red wine spoilage induced by Brettanomyces yeast. Sens. Actuators B Chem. 2007, 124, 167–171. [Google Scholar] [CrossRef]
- Jaiswal, R.; Patras, M.A.; Eravuchira, P.J.; Kuhnert, N. Profile and characterization of the chlorogenic acids in green Robusta coffee beans by LC-MS n: Identification of seven new classes of compounds. J. Agric. Food Chem. 2010, 58, 8722–8737. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Wight, J. The measurement of feruloylquinic acids and caffeoylquinic acids in coffee beans. Development of the technique and its preliminary application to green coffee beans. J. Sci. Food Agri. 1976, 27, 73–84. [Google Scholar]
- Serra, R.; Grande, R.; Butrico, L.; Buffone, G.; Caliò, F.G.; Squillace, A.; Rizzo, B.A.; Massara, M.; Spinelli, F.; Ferrarese, A.G. Effects of a new nutraceutical substance on clinical and molecular parameters in patients with chronic venous ulceration. Intl. Wound. J. 2016, 13, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Variyar, P.S.; Bandyopadhyay, C. Estimation of phenolic acids in cinnamon, clove, cardamom, nutmeg and mace by high performance liquid chromatography. J. Spic. Arom. Crops. 1995, 4, 129–134. [Google Scholar]
- Pura Naik, J.; Jagan Mohan Rao, L.; Mohan Kumar, T.; Sampathu, S. Chemical composition of the volatile oil from the pericarp (husk) of large cardamom (Amomum subulatum Roxb.). Flav. Frag. J. 2004, 19, 441–444. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Karbancioglu-Guler, F. Cardamom, Cumin, and Dill Weed Essential Oils: Chemical Compositions, Antimicrobial Activities, and Mechanisms of Action against Campylobacter spp. Molecules 2017, 22, 1191. [Google Scholar] [CrossRef] [Green Version]
- Leyva-López, N.; Nair, V.; Bang, W.Y.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of terpenes and polyphenols from three species of Oregano (Lippia graveolens, Lippia palmeri and Hedeoma patens) on the suppression of lipopolysaccharide-induced inflammation in RAW 264. 7 macrophage cells. J. Ethnopharmacol. 2016, 187, 302–312. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Bang, W.Y.; Nair, V.; Angulo-Escalante, M.A.; Cisneros-Zevallos, L.; Heredia, J.B. Protective role of flavonoids and lipophilic compounds from Jatropha platyphylla on the suppression of lipopolysaccharide (LPS)-induced inflammation in macrophage cells. J. Agric. Food Chem. 2016, 64, 1899–1909. [Google Scholar] [CrossRef]
- Nair, V.; Bang, W.Y.; Schreckinger, E.; Andarwulan, N.; Cisneros-Zevallos, L. Protective Role of Ternatin Anthocyanins and Quercetin Glycosides from Butterfly Pea (Clitoria ternatea Leguminosae) Blue Flower Petals against Lipopolysaccharide (LPS)-Induced Inflammation in Macrophage Cells. J. Agric. Food Chem. 2015, 22, 6355–6365. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Bang, W.Y.; Delgadillo-Puga, C. Ellagic Acid and Urolithins A and B Differentially Regulate Fat Accumulation and Inflammation in 3T3-L1 Adipocytes While Not Affecting Adipogenesis and Insulin Sensitivity. Int. J. Mol. Sci. 2020, 18, 2086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Polyphenolics from peach (Prunus persica var. Rich Lady) inhibit tumor growth and metastasis of MDA-MB-435 breast cancer cells in vivo. J. Nutr. Biochem. 2014, 25, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Delgadillo-Puga, C.; Noriega, L.G.; Morales-Romero, A.M.; Nieto-Camacho, A.; Granados-Portillo, O.; Rodríguez-López, L.A.; Alemán, G.; Furuzawa-Carballeda, J.; Tovar, A.R.; Cisneros-Zevallos, L.; et al. Goat’s Milk Intake Prevents Obesity, Hepatic Steatosis and Insulin Resistance in Mice Fed A High-Fat Diet by Reducing Inflammatory Markers and Increasing Energy Expenditure and Mitochondrial Content in Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 5530. [Google Scholar] [CrossRef] [PubMed]










| COX2 | Fw-ACATCGATGTCATGGAACTG Rv-GGACACCCCTTCACATTATT |
| IL-6 | Fw-TGACAACCACGGCCTTCCCT Rv-AGCCTCCGACTTGTGAAGTGGT |
| LXRα | Fw-AAGCCCTGCATGCCTACGT Rv-TGCAGACGCAGTGCAAACA |
| β-actin | Fw-CCCAGGCATTGCTGACAGG Rv-TGGAAGGTGGACAGTGAGGC |
| TNFα | Fw-ACTGGCAGAAGAGGCACTCC Rv-CGATCACCCCGAAGTTCA |
| PPARγ | Fw-GATGCACTGCCTATGAGCACTT Rv-AGAGGTCCACAGAGCTGATTCC |
| NFKβ | Fw-GGTGGAGGCATGTTCGGTA Rv-TGACCCCTGCGTTGGATT |
| Peak No. | RT (min) | M-H | * MS Fragments | Identification | Seed (mg/100 g) | Skin (mg/100 g) | Whole Cardamom (mg/100 g) |
|---|---|---|---|---|---|---|---|
| 1 | 10.70–10.78 | 153 | 109 | Protocatechuic acid | 29.69 | 6.09 | 23.48 |
| 2 | 14.00–14.33 | 153 | 109, 103 | Gentisic acid | 1.98 | 3.87 | 1.14 |
| 3 | 18.20–18.21 | 179 | 161, 135 | Caffeic acid | 26.23 | 18.73 | 29.51 |
| 4 | 18.25–18.35 | 197 | 191, 173 | Syringic acid | 36.43 | 6.69 | 34.11 |
| 5 | 19.16–19.22 | 193 | 179 | Ferulic acid | 6.68 | 4.11 | 8.51 |
| 6 | 19.78–18.02 | 167 | 135, 121 | Vanillic acid | 4.28 | 7.04 | 4.99 |
| 7 | 20.8–21.19 | 353 | 190, 179 | 5-O-caffeoylquinic acid | 14.6 | 5.29 | 28.96 |
| 8 | 22.6–22.8 | 163 | 143, 135, 121 | p-coumaric acid | 0.44 | 1.61 | 3.53 |
| 9 | 23.08–24.8 | 397 | 191, 173 | Sinapoyl quinic acid | 1.46 | 1.99 | 5.79 |
| 10 | 25.31–27.31 | 367 | 173, 161 | Feruloyl quinic acid | 0.054 | 0.27 | 0.97 |
| 11 | 31.3–31.34 | 609 | 447, 301 | Rutin | 2.69 | 16.02 | 0.57 |
| Peak No. | Compound | Mass | Retention Time | Seed (% Area) | Skin (% Area) | Whole Cardamom (% Area) |
|---|---|---|---|---|---|---|
| 1 | Cis-α-Terpineol | 154 | 27.17 | 7.99 | 4.4 | 8.12 |
| 2 | Cis-β-Terpineol | 154 | 27.57 | 1.43 | 0.84 | 1.55 |
| 3 | Linalool | 154 | 33.66 | 0.84 | 0.83 | 0.92 |
| 4 | Terpinen-4-ol | 154 | 34.94 | 0.23 | 6.42 | 0.33 |
| 5 | α-Terpineol | 154 | 36.22 | 0.92 | 1.31 | 0.98 |
| 6 | Geraniol | 154 | 37.57 | 1.05 | 2.2 | 1.22 |
| 7 | Trans-Nerolidol | 222 | 38.15 | 0.47 | 1.78 | 0.61 |
| 8 | α-Acorenol | 222 | 39.39 | 0.52 | 1.55 | 0.64 |
| 9 | Cubebol | 222 | 39.78 | 0.26 | 1.61 | 0.28 |
| 10 | Isopathulenol | 220 | 41.64 | 0.26 | 1.45 | 0.32 |
| 11 | Globulol | 222 | 42.24 | 0.75 | 1.64 | 0.79 |
| 12 | Trans-Farnesol | 222 | 43.91 | 0.62 | 1.18 | 0.73 |
| 13 | Ambrial | 234 | 50.49 | 5.3 | 1.52 | 5.35 |
| 14 | Eicosane | 282 | 51.21 | 0.22 | 5.53 | 0.28 |
| 15 | Costunolide | 232 | 54.43 | 68.11 | 29.8 | 69.43 |
| 16 | Coronarin | 300 | 54.85 | 1.12 | 2.73 | 1.25 |
| 17 | β-Mono Palmitin | 330 | 50.59 | 0.23 | 0.58 | 0.28 |
| 18 | β-Mono Stearin | 358 | 56.65 | 0.39 | 0.15 | 0.43 |
| 19 | Erucylamide | 337 | 62.71 | 0.41 | 0.23 | 0.48 |
| 20 | β-Sitosterol | 414 | 64.17 | 0.84 | 0.18 | 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sreedharan, S.; Nair, V.; Cisneros-Zevallos, L. Protective Role of Phenolic Compounds from Whole Cardamom (Elettaria cardamomum (L.) Maton) against LPS-Induced Inflammation in Colon and Macrophage Cells. Nutrients 2023, 15, 2965. https://doi.org/10.3390/nu15132965
Sreedharan S, Nair V, Cisneros-Zevallos L. Protective Role of Phenolic Compounds from Whole Cardamom (Elettaria cardamomum (L.) Maton) against LPS-Induced Inflammation in Colon and Macrophage Cells. Nutrients. 2023; 15(13):2965. https://doi.org/10.3390/nu15132965
Chicago/Turabian StyleSreedharan, Shareena, Vimal Nair, and Luis Cisneros-Zevallos. 2023. "Protective Role of Phenolic Compounds from Whole Cardamom (Elettaria cardamomum (L.) Maton) against LPS-Induced Inflammation in Colon and Macrophage Cells" Nutrients 15, no. 13: 2965. https://doi.org/10.3390/nu15132965