 J Clin Aesthet Dermatol. 2020;13(6):24–34
J Clin Aesthet Dermatol. 2020;13(6):24–34
by George Sadowski, MD and Julian Sadowski
Dr. G. Sadowski and J. Sadowski are employees of Nourishing Biologicals LLC in St. Augustine, Florida.
FUNDING: Funding provided for this study by Nourishing Biologicals LLC.
DISCLOSURES: Both authors are employees of Nourishing Biologicals LLC.
ABSTRACT: Background. Due to both intrinsic and extrinsic damage, the skin is where easily noticable signs of aging manifest.
Objective. We sought to assess the effects of two complex novel topical formulations, L’Unique Miracular Facial Serum (LMFS) and L’Unique Skin Essence (LSE) (Nourishing Biologicals LLC, St. Augustine, Florida) on hydration, firmness, elasticity, wrinkling, and pore size of facial skin after initial application and then after four, eight, and 12 weeks of use.
Methods. An open-label study was conducted on subjects (N=32) between the ages of 45 and 65 years (mean: 57 years). Subjects were treated with a twice-daily application of LMFS and LSE for a total of 12 weeks following a one-week washout period. The test products were gently applied in a circular motion to the face each morning and evening. Measurements of skin hydration, transepidermal water loss (TEWL), and skin elasticity and firmness and three-dimensional skin surface evaluations were performed at each visit. Skin lift and pore size assessments were also completed using clinical photography. Subjective outcomes were assessed by a posttreatment product efficiency survey at the end of each visit.
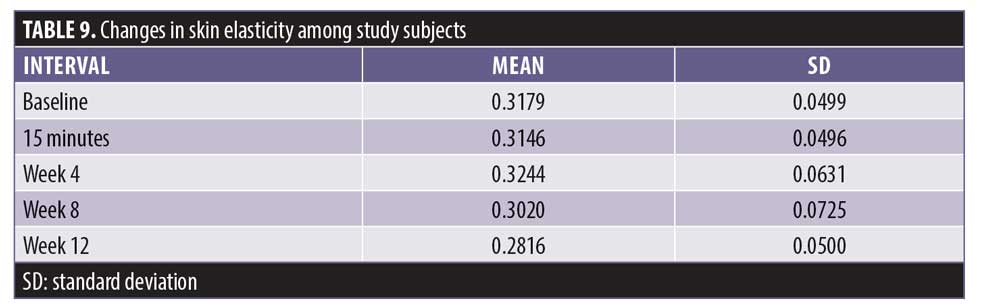
Results. Objective instrumental measurements showed statistically significant improvements in skin hydration (20.19%), TEWL (25.96% at 15 minutes), firmness (24.77%), skin elasticity (11.40%), and skin lift (5.41%) with product use. Improvements in pore size and wrinkle depth were not statistically significant.
Conclusion. Use of the test products produced significant improvements in skin hydration, TEWL, firmness, and skin elasticity with associated improvements in facial skin appearance.
Keywords: Skin hydration, skin firmness, skin elasticity, facial appearance, topical skin cream, anti-aging skin treatment
Aging is caused by free radical destruction of proteins, lipids, deoxyribonucleic acid (DNA), ribonucleic acid (RNA), organelles, and other vital structures. Under normal conditions, damage by free radicals is repaired by antioxidants, but these unfortunately decline with age, increasing the likelihood of continuous damage. Oxidative stress occurs when there is a loss of balance between the damage caused by free radicals or reactive chemical species and cellular antioxidant self-repair. This balance favors oxidation as small, nontoxic amounts of reactive oxidative species are required for cellular communication, immune system function, and control of the cell life cycle, which leads to beneficial cellular adaptation and balance.1–7
Because of its large surface area and contact with the external environment, the skin also receives damage from free radicals generated by external factors, such as air pollutants, smoking, changes in humidity and temperature, ionizing and nonionizing radiation from sunlight, ultraviolet radiation, elevated concentrations of ozone, various forms of particulate matter, and other environmentally induced agents.8–10
Photo-oxidative stress induces deranged cellular communication, proinflammatory processes, and altered gene expression and stimulates the production of end-glycation products (AGES) and other destructive enzymes.11–16 Because the skin ages from both intrinsic and extrinsic causes, the damage is cumulative and becomes more pronounced over time, especially on sun-exposed areas, such as the face.17–19
The visibility of the skin represents a unique organ system that facilitates inspection following application of topical formulations. The scientific literature is replete with evidence supporting the beneficial effects of many minerals, vitamins, antioxidants, anti-inflammatories, and growth factors in terms of skin hydration and firmness.20–86
Due to the multifactorial causes of skin aging, multiple ingredients can be strategically employed in creating anti-aging formulations, as these can play a direct or facilitative role. Combining multiple ingredients together is a logical step to achieve greater results, but formulators must also determine the safety and effectiveness of these formulations.
Here, we describe and evaluate a formulation developed using a combination of numerous active antioxidants, anti-inflammatories, natural oils, minerals, polysaccharides, vitamins, and growth factors designed to improve the skin appearance.
Methods
Patch safety test. A repeat insult patch safety test was performed to determine the irritation and sensitization (i.e., contact allergy) potential of test materials. The study was conducted by an independent laboratory (BioScreen Testing Services Inc., Phoenix, Arizona). Fifty-five subjects between the ages of 18 and 62 years with Fitzpatrick Skin Types III and IV were selected.
Test materials consisting of L’Unique Miracular Facial Serum (LMFS) and L’Unique Skin Essence (LSE) (Nourishing Biologicals LLC, St. Augustine, Florida) (Tables 2 and 3) were evaluated under occlusive conditions. An 8-mm aluminum Finn chamber (Epitest Ltd., Tuusula, Finland) supported on Scanpor Tape (Norgesplaster A/S, Kristiansand, Norway) was used. Approximately 0.02 to 0.05 of liquid or 0.02 to 0.05gm of solid were applied and rubbed into the skin in the infrascapular areas. The patches were applied directly over these areas and remained for 48 hours after the initial application. All subsequent applications remained for 24 hours. The procedure was repeated until nine consecutive 24-hour exposures had been conducted three times a week for three consecutive weeks. Test sites were evaluated by trained laboratory personnel and any adverse reactions were reported. No irritation or sensitivity effects were reported.
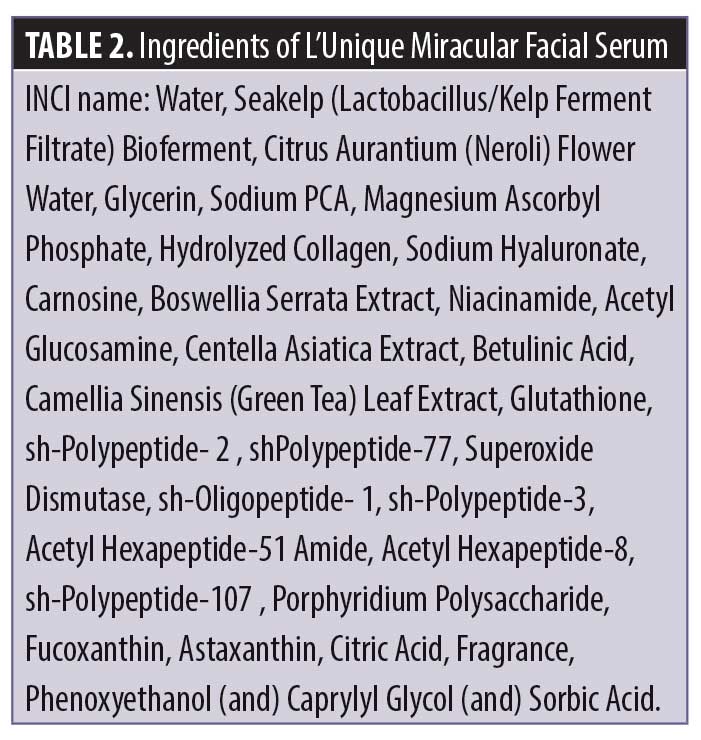

Efficacy evaluation. Separately, a 12-week, open-label clinical study was conducted to test the effectiveness of LMFS and LSE with regard to skin hydration/moisturization, skin barrier function, skin firmness, skin elasticity, skin lift, effect on fine lines and wrinkles, and improvement in pore size.
The conduct of this study complied with the International Conference of Harmonization Tripartite Guideline on Good Clinical Practice, applicable United States Food and Drug regulations/guidelines set forth in 21 CFR Parts I and 50, and the standard practices of BioScreen Testing Services. The study was conducted over a 13-week period including, one week of washout followed by 12 weeks of product usage. Written informed consent and photoconsent was obtained by all participants. Subjects were evaluated for study eligibility at Visit 1 (screening, selection, and clinical baseline evaluation), Visit 2 (Week 4), Visit 3 (Week 8), and Visit 4 (Week 12). Panel selection was accomplished by advertisements in the Denver, Colorado area. Individuals were admitted into the study based on medical history and findings during the prestudy interview and examination. Thirty-five healthy women aged 45 to 65 years (mean: 57 years) with skin photoaging and chronoaging meeting the inclusion criteria were enrolled (Table 1). Of the total participants enrolled, 76 percent of subjects had Fitzpatrick Skin Type III, 21 percent had Fitzpatrick Skin Type IV, and three percent had Fitzpatrick Skin Type V. By race, the study population included 26 Caucasian participants, one African American participant, and seven Hispanic participants.
 The antiaging regimen, comprising LMFS and LSE, was applied to the entire face twice daily—once in the morning and once in the evening—after cleansing. One to two pumps of the LMFS were applied onto dry skin and massaged into the entire face and neck, then allowed to dry. Separately, one or two pumps of LSE were applied onto the entire face and neck and massaged into the skin and allowed to dry.
The antiaging regimen, comprising LMFS and LSE, was applied to the entire face twice daily—once in the morning and once in the evening—after cleansing. One to two pumps of the LMFS were applied onto dry skin and massaged into the entire face and neck, then allowed to dry. Separately, one or two pumps of LSE were applied onto the entire face and neck and massaged into the skin and allowed to dry.
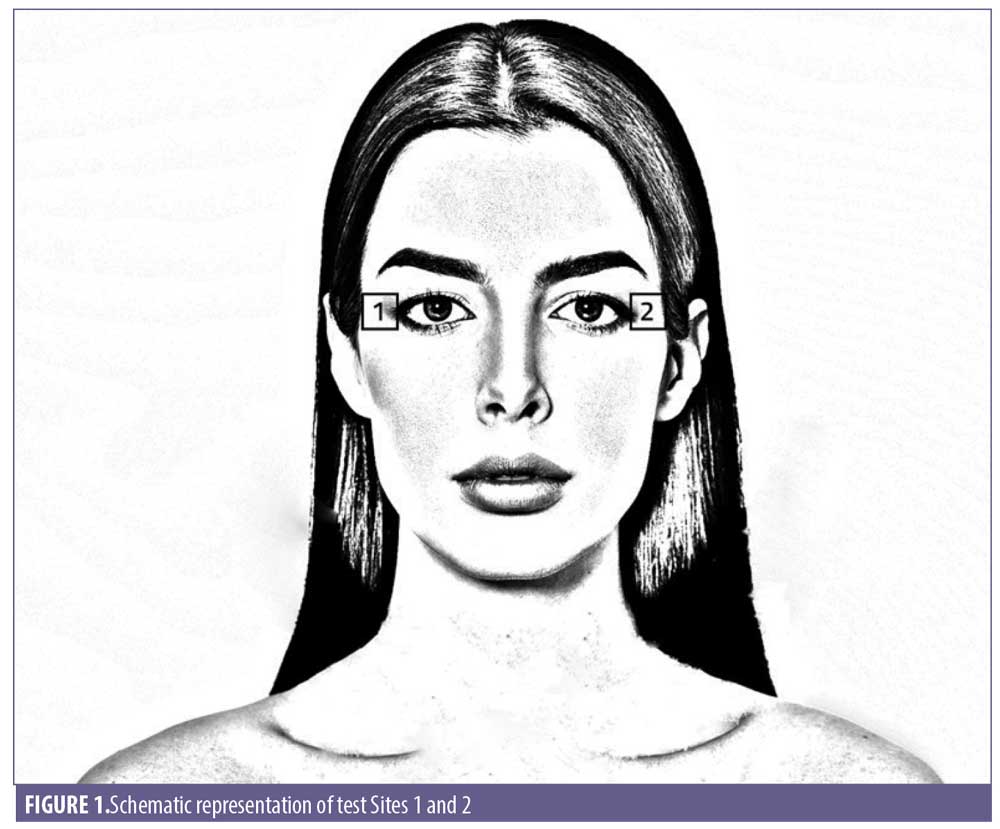
Changes in skin conductance, impedance or capacitance were used to study epidermal hydration in vivo. The Corneometer CM 825 (Courage and Khazaka, Cologne, Germany) and Dermalab Series SkinLab Combo Moisture probe (Cortex Technology, Hadsund, Denmark) were used to measure the electrical capacitance and conductance, respectively, of the skin. Three replicate measurements were taken on two designated test sites (Figure 1) at each interval.87,88
Experimental technique. Transepidermal water loss (TEWL) is a measure of skin barrier function. The evaporimeter probe with two sensors was used to measure the vapor pressure gradient arising within the chamber and between the skin and the surrounding air. TEWL was measured using the DermaLab Evaporimeter (Cortex Technology, Hadsund, Denmark) or AquaFlux Model AF2OO (Biox Systems Ltd., London, United Kingdom).89 An absence of change in TEWL at posttreatment intervals relative to baseline indicates the mildness of the treatment, in that it has not disturbed barrier function. Decreases in TEWL indicate an improvement in skin barrier function such that less water is lost through the skin barrier. One TEWL measurement was taken at designated treatment sites at each measurement interval.
Separately, the Cutometer measures viscoelastic properties of the skin in vivo. The Cutometer MPA 580 (Courage and Khazaka) was used to measure skin firmness and elasticity. The application of negative pressure draws the skin into the aperture of the probe. The resistance of the skin to negative pressure (i.e., firmness) and its ability to return to its original position (i.e., elasticity) are displayed. The data were analyzed using the device software.90–92
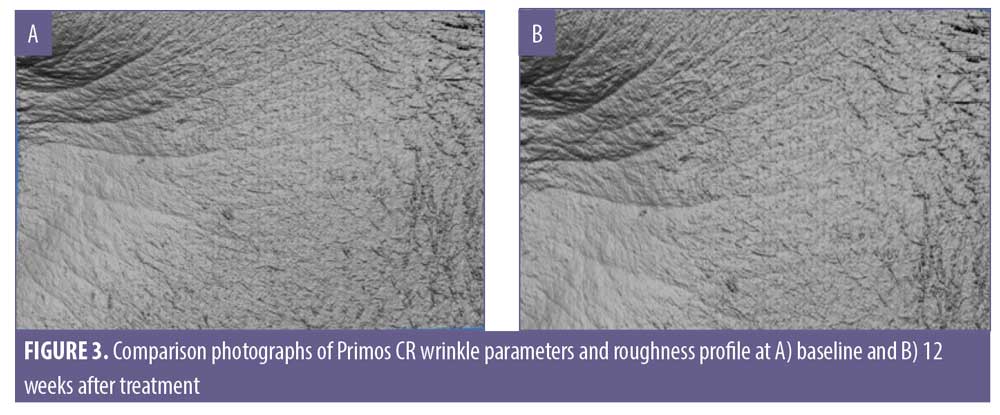
The Primos-CR (Canfield Scientific, Parsippany-Troy Hills, New Jersey) was used to evaluate the three-dimensional surface of the skin. The Primos was mounted on an adjustable stand to allow for modification on a variety of axes to maximize the surface area that was aligned with the sensor’s focal plane. Data files captured by the Primos-CR were analyzed using the Primos software for wrinkle parameters average depth of wrinkles, total wrinkle volume (Ra, the average of the absolute values of the profile heights of the roughness profile and RZ, the average of the single roughness depths).
Photographs were taken in accordance with regulations provided by consumer protection agencies, such as the Federal Trade Commission (FTC), the Food and Drug Administration (FDA), and other regulatory authorities. The following guidelines were followed: 1) head position was the same in before and after photos; and 2) the same lighting conditions and distance from the camera were used for both before and after pictures.
Clinical photographs of faces of the subjects (frontal, left lateral, and right lateral) were taken and evaluated with the Canfield VISIA CR system using the Standard I modalities. Photographs obtained were evaluated for the appearance of skin lift using the following scale (half-point increments were allowed): 0=none, 1–3=mild, 4–6=moderate, 7–9=severe. Photographs obtained were evaluated for the appearance of pore size using the VAESTRO Image Analysis Toolkit. The Vaestro and Visia CR image analysis toolkit are commonly used to assess the effect of test products on pore size.93
Each subject completed a self-assessment questionnaire following the immediate, 24-hour, four-week, eight-week, and 12-week posttreatment intervals. The choice of answers was limited to “yes” or “no.”
Procedure. Subjects reported to the facility a minimum of five days prior to the start of the study to sign an informed consent form, Health Insurance Portability and Accountability Act form, Code of Conduct form, and photography release form. Subjects were interviewed and a medical history was obtained. Enrolled subjects began a one-week washout period. Subjects were given specific instructions prohibiting the use of any other personal care products for the washout period and study duration with the exception of those provided. Any current facial makeup was excluded if it contained any antiaging properties. Subjects were instructed to refrain from introducing any new cosmetics during the duration of the study and not to wear any type of cosmetic during study visits. Following the five-day washout period, subjects returned to the testing facility. All subjects were instructed to wash their face with a supplied standardized neutral cleanser and gently pat dry with a paper towel.
Following equilibration, the baseline (i.e., pretreatment) evaluation included:
Close-up facial photographs (left lateral, right lateral, and frontal)
Primos measurements at Sites 1 and 2
Skin hydration measurements at Sites 1 and 2
Skin barrier function measurements at Sites 1 and 2
Skin firmness and elasticity measurements at Sites 1 and 2
Following baseline measurements, subjects were instructed to use the test product regimen according to provided use instructions. During this step, the subjects remained in the exam room. Then, at 15 minutes posttreatment, the subjects underwent the following additional procedures/measurements performed by trained BCS staff:
Skin hydration measurements at Sites 1 and 2
Skin barrier function measurement at Sites 1 and 2
Skin firmness and elasticity measurements at Sites 1 and 2
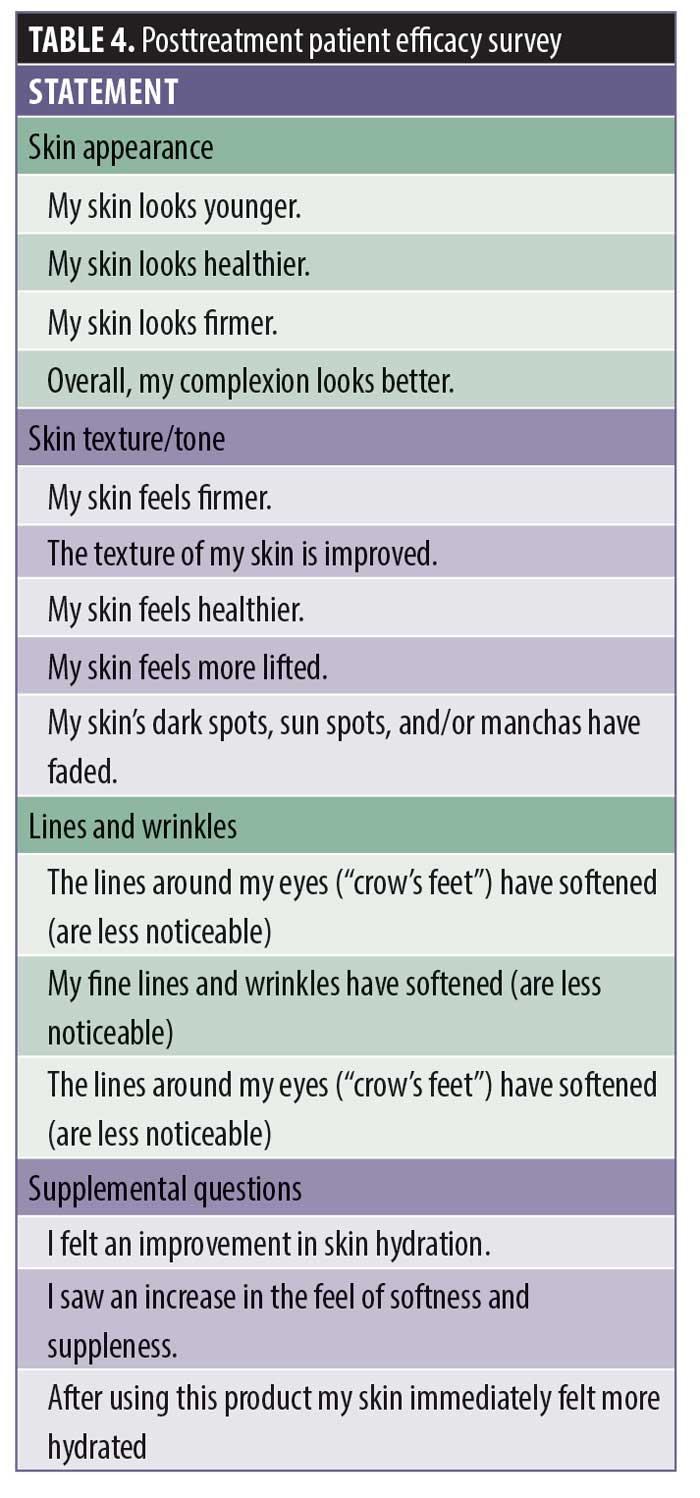
Posttreatment product efficiency survey (Table 4)
 Next, subjects were dismissed from the testing facility and informed to return 24 hours after this initial treatment. During this time, subjects were instructed not to wet or apply any products (e.g., lotions, creams, or cleansers) to their face until after the 24-hour measurements. At 24 hours posttreatment, subjects were assembled, remaining quietly seated for a minimum of 20 minutes in a room maintained at approximately 20°C to 24°C and 30- to 50-percent relative humidity. Temperature and humidity were maintained and recorded during subject testing. This protocol was repeated at Weeks 4, 8, and 12 posttreatment.
Next, subjects were dismissed from the testing facility and informed to return 24 hours after this initial treatment. During this time, subjects were instructed not to wet or apply any products (e.g., lotions, creams, or cleansers) to their face until after the 24-hour measurements. At 24 hours posttreatment, subjects were assembled, remaining quietly seated for a minimum of 20 minutes in a room maintained at approximately 20°C to 24°C and 30- to 50-percent relative humidity. Temperature and humidity were maintained and recorded during subject testing. This protocol was repeated at Weeks 4, 8, and 12 posttreatment.
At the 24-hour posttreatment evaluation, subjects had the two measurements performed by trained BioScreen staff:
Skin barrier function measurements at Sites 1 and 2
Posttreatment questionnaire was collected
At the Weeks 4, 8, and 12 posttreatment evaluations, the following were performed:
Close-up facial photographs (left lateral, right lateral, and frontal)
Primos measurements at Sites 1 and 2
Skin hydration measurements at Sites 1 and 2
Skin firmness and elasticity measurements at Sites 1 and 2
Posttreatment questionnaire
Adverse events. Any adverse events consisting of any untoward medical occurrence—whether or not considered to be study-related, a disease, an exacerbation of a pre-existing illness or condition, an occurrence of an intermittent illness or condition, a set of related symptoms or signs, or a single symptom or sign—were promptly recorded and sufficiently documented. All adverse events were monitored until they resolved, stabilized, the subject was lost to follow-up, or the event was otherwise explained.
Subject discontinuation. Strict adherence to the study was monitored by BioScreen investigators at each interval visit. Criteria for the discontinuation of a subject from participating in the study included the following:
Significant protocol violation
Serious adverse experience
Request of the subject
Any unmanageable factor that, in the investigator’s opinion, could significantly interfere with the protocol or interpretation of results
Data analysis. Statistical analyses tested the hypothesis that the pretreatment values of each parameter were statistically different from the posttreatment values. Statistical analysis was performed using a z-test. Statistical significance was declared if the two-tailed p-value was greater than 0.05.
Results
A total of 32 healthy women, aged 45 to 65 years, with an average age of 57 years completed the present clinical study evaluating the effectiveness of LMFS and LSE for improving skin conditions and efficacy of anti-aging skin care. Sensitivity and/or irritability testing using a patch test result did not reveal any sensitivity or irritation from the test products and confirmed the safe use of test products in the clinical study.
The clinical study was conducted from October 11, 2018, to January 18, 2018, in Arizona during an expected drop in temperature conducive to skin dryness. The results of clinical efficacy grading indicated that the use of this antiaging regimen for 12 weeks produced a statistically significant improvement in face hydration, skin barrier function, skin firmness, and skin lift. Conversely, measurements of the diminution of pore size and fine lines and wrinkles of the eye area did not reach statistical significance despite positive trending.
There was a statistically significant improvement in skin hydration as measured by the Corneometer from baseline at 15 minutes posttreatment and at the four-week, eight-week, and 12-week posttreatment intervals (Table 5). A statistically significant percentage of subjects demonstrated an improvement in skin hydration from baseline at the 15-minute, four-week, eight-week, and 12-week posttreatment intervals (Table 6). All of the subjects experienced the improvement in hydration starting with the first application; this continued during the course of the study.
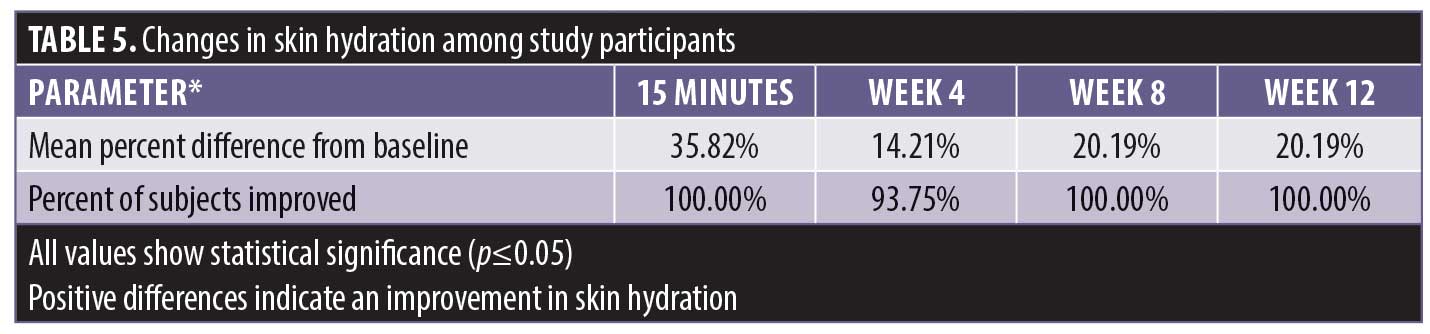
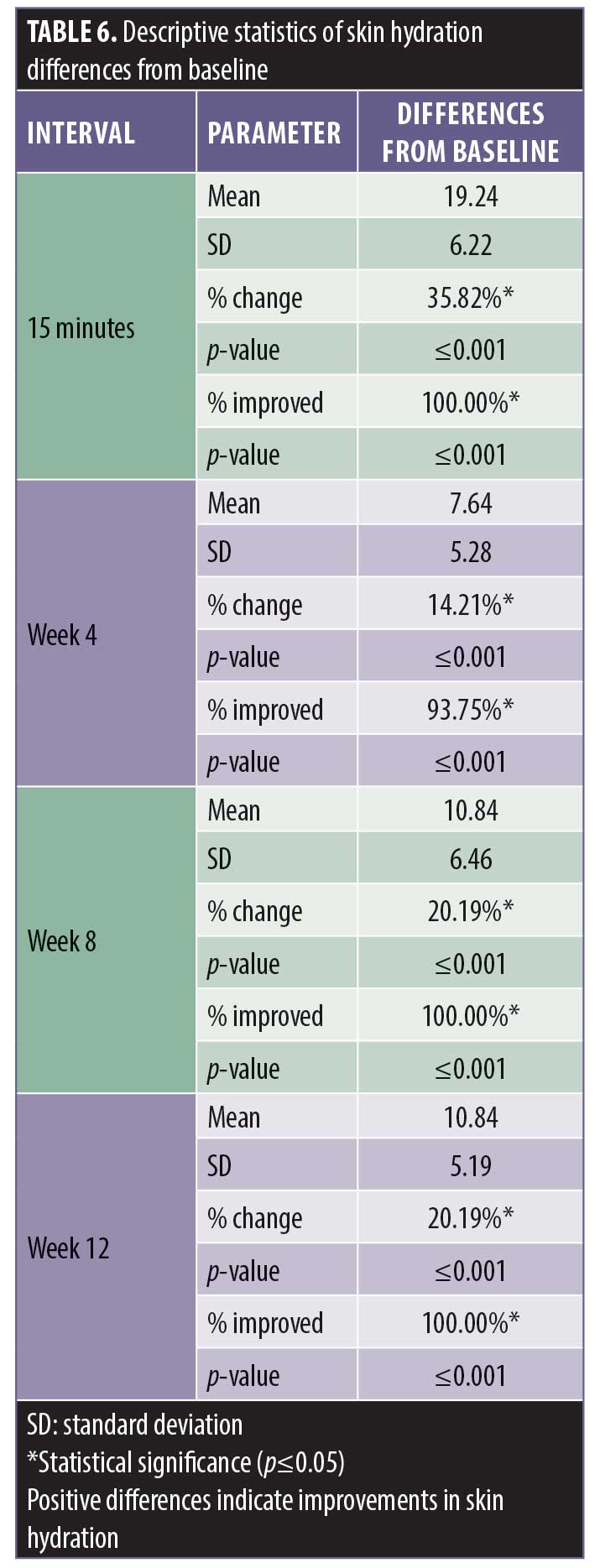
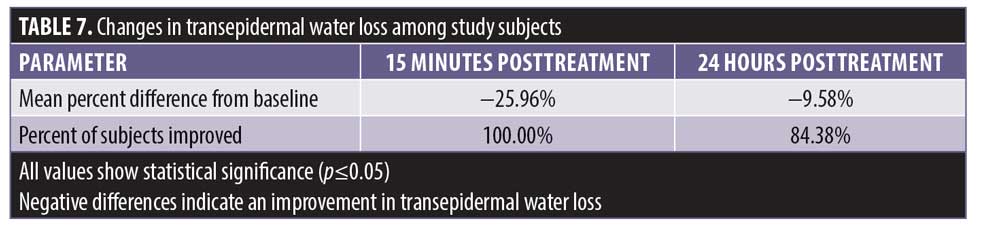
There was a statistically significant decrease in TEWL, indicating improvements in skin barrier function, from baseline at 15 minutes posttreatment and at 24 hours prior to treatment (Table 7). A statistically significant number of subjects demonstrated an improvement in skin barrier function from baseline at 15 minutes posttreatment and 24 hours prior to treatment. The 15-minute posttreatment measurements showed greatly improved hydration in all subjects. Hydration declined over 23 hours following treatment with significant improvement in 84.3 percent of the subjects.
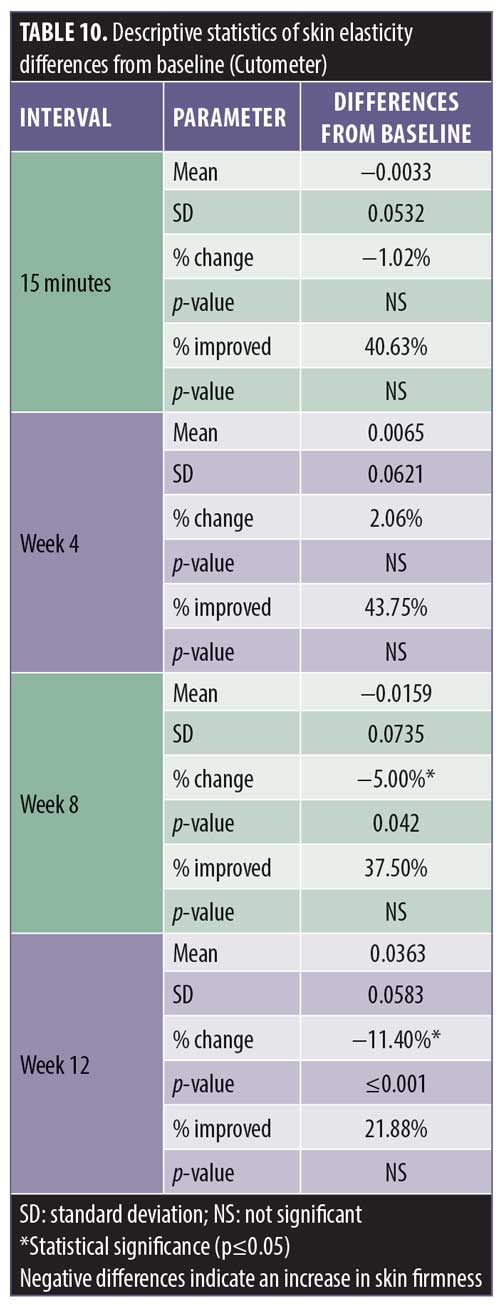
Cutometer measurements showed a statistically significant improvement in skin firmness from baseline at 15 minutes, four weeks, eight weeks, and 12 weeks posttreatment (Table 8). The improvement was incremental, starting at -4.04 percent at 15 minutes after treatment and continuing to increase to a maximum of -24.7 percent at Week 12.
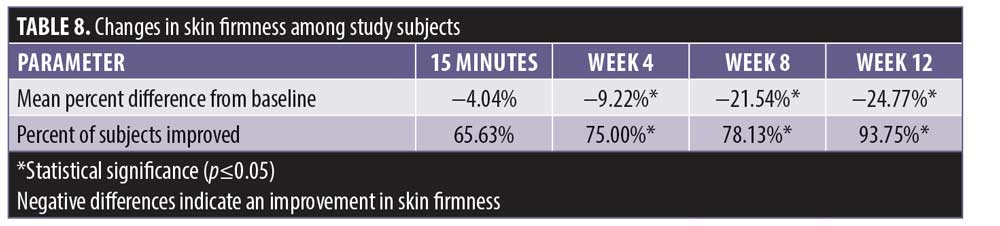
Additionally, 93.7 percent of the subjects experienced a maximal improvement in skin firmness. A negative difference in skin elasticity is a measurement of increasing skin firmness. The skin elasticity decreased incrementally up to -11.4 percent at Week 12, with the interval between Weeks 8 and 12 demonstrating the most improvement (Tables 9 and 10).
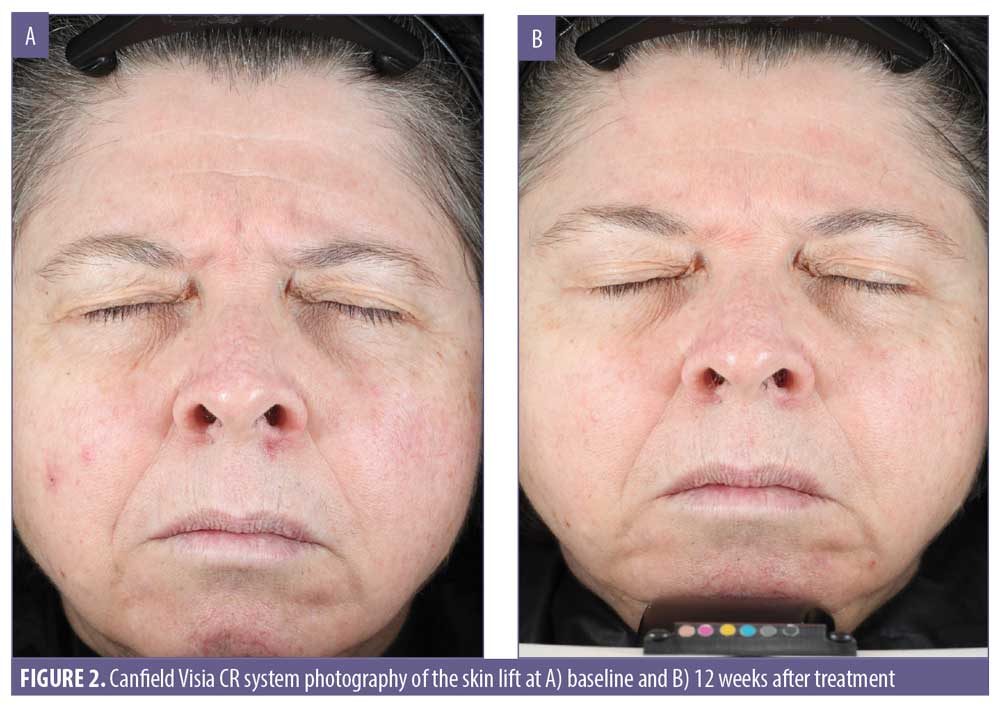
Clinical photography for the expert grading of the appearance of skin lift was accomplished using the Canfield VISIA CR system. This showed a statistically significant improvement in the appearance of skin lift from baseline at four weeks, eight weeks, and 12 weeks posttreatment (Table 11). The improvement in skin lift was limited to 43.7 percent of the subjects.

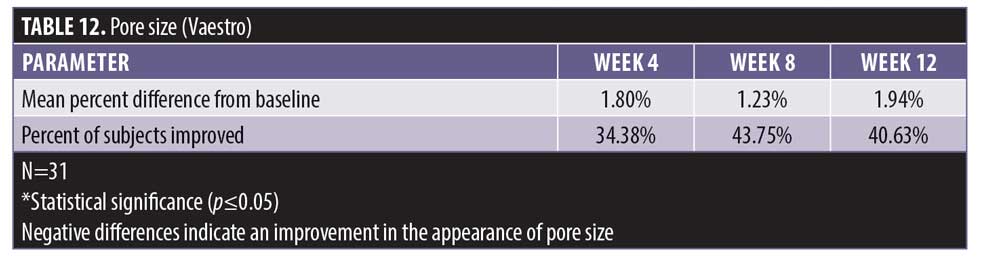
There was no statistically significant change in the appearance of pore size from baseline at four weeks, eight weeks, and 12 weeks posttreatment when using the Vaestro Image Analysis Toolkit (Table 12). Despite the lack of statistical power, an improvement in pore size was experienced by 40.6 percent of the subjects.
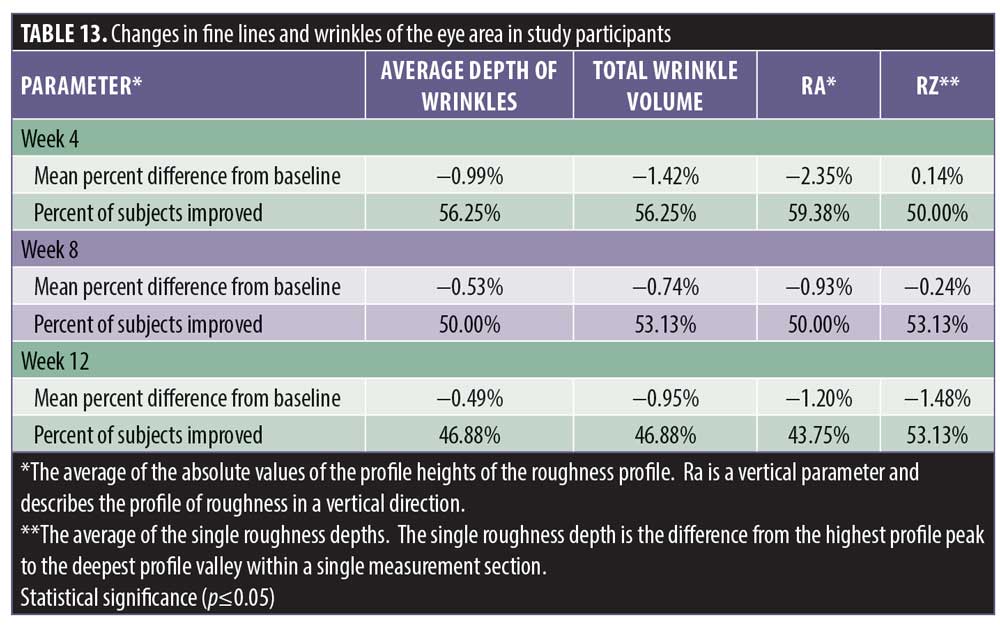
There was no statistically significant change in the appearance of fine lines and wrinkles of the eye area from baseline at four weeks, eight weeks, and 12 weeks posttreatment despite positive trending in the average depth of wrinkles with an improvement noted in 46.8 percent of the subjects (Table 13). The reduction of wrinkle depth progressed from -0.99% to -0.49% (49% reduction). Wrinkle volume was reduced from 1.42% to 0.95% in 46.8 percent of the subjects.
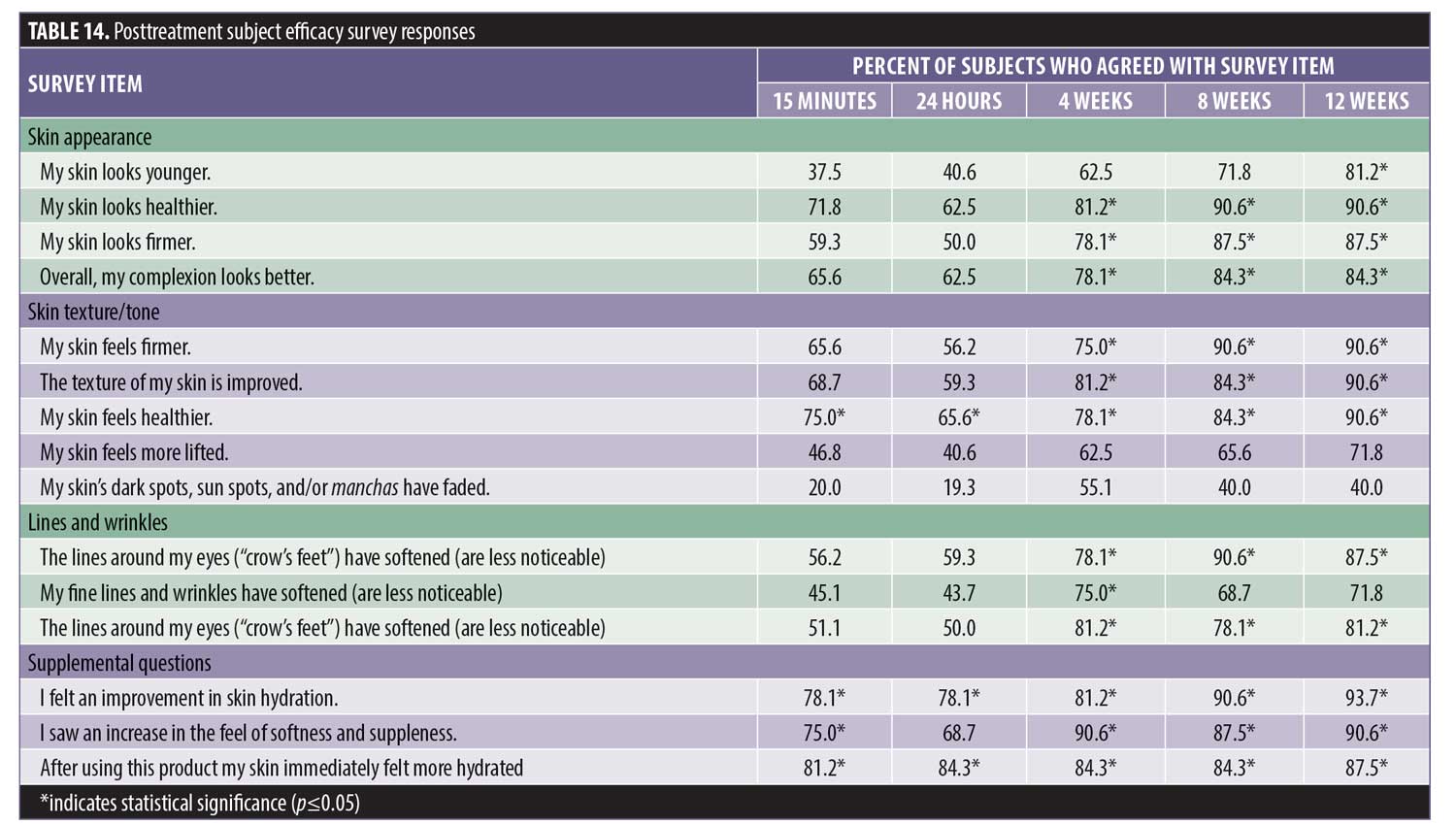
Subject efficacy survey responses. Posttreatment product efficacy survey results were collected at each visit. The number of positive responses increased with product usage. Subjects reported hydration/moisture improved by 78 percent following a single application, which increased to 93.7 percent at 12 weeks. Skin texture, smoothness, and firmness improved to 90.6 percent at 12 weeks. Approximately 90.6 percent of subjects felt that their skin looked softer and healthier, while a majority believed that their fine lines and wrinkles (87.5%), deep lines and wrinkles (71.8%), and lines around the eyes (81.2%) were less noticeable. Most felt that their skin appeared younger (81.2%), healthier (90.6%), and more lifted (71.8%). Most (84.3%) indicated that their complexion looked better.
Discussion
Skin aging is an external manifestation of a multifactorial process causing a progressive loss in hydration, elasticity, firmness, and skin tone from both intrinsic and extrinsic causes. The novel formulation of nutraceuticals and peptide growth factors evaluated in this study have demonstrated the ability to improve skin hydration, elasticity, wrinkle formation, and skin tone.
The discovery and development of growth factors and active peptides has advanced the potential for the direct treatment of the causes of skin aging. The growth factor mechanism of action can be selected to bear its maximal effect on a causative metabolic process in need of support. Naturally occurring human growth factors serve as cellular chemical messengers directing cellular growth, migration, extracellular matrix activity, and the inflammatory response.69–73 Numerous actives, including peptides, are included in the test formulations.
Active peptides consist of short amino-acid residues (3–20 amino acids) with specific sequences and biological functions that can stimulate anabolic cellular metabolism. They contain the active portions that give them the activity of larger molecules synthesized by the organism. Their small size facilitates better penetration into the skin. They can have a varied amino acid composition, chemical structure, and three-dimensional conformation. Because of this, a large number of possible active peptides can be synthesized with protein-binding specificity. Many have been shown to possess antimicrobial, antioxidant, and anti-inflammatory activities suitable for the treatment of chronic disease.37–39,52–82
These peptides have been classified as neurotransmitter affecting, signaling, topically protective, or as carrier peptides. Some synthetic peptides have been formulated to stimulate the production of collagen, elastin, proteoglycans, glycosaminoglycans, and fibronectin, improving skin elasticity and appearance.42–49
Peptides are suitable for topical application since injection can illicit an immune response and ingestion is unlikely to be useful due to the breakdown by digestive enzymes. Since human peptides in the skin decrease with aging, the topical application of peptides in the area where they are needed most to preserve skin health is logical and avoids systemic complications. Some have already been introduced in cosmetics and their effects are limited to varied claims of improvement in skin appearance.
The topical application of a combination of nutraceuticals, active peptides, and growth factors contained in these novel formulations are generally considered as safe individually and our experience supports the absence of any adverse effects. No skin sensitivity or irritation was experienced by any of the participants (N=55) during a three-week repeat insult patch safety test or during the 12-week clinical study.
The impact of objective testing is instrumental in conveying a scientifically sustainable improvement. The objective measurement of improved skin hydration achieved statistical significance. Since skin hydration is important to skin health, its moisturizing or occlusive effects improve the health of the skin and may impact skin aging. The objective measurements showed a 35-percent improvement in skin hydration at 15 minutes after a single treatment. This was followed by a 14-percent improvement from baseline at 24 hours postapplication at Week 4 and a 20-percent improvement 24 hours postapplication at Week 8. This effect was observed in all of the subjects.
The improvements in skin firmness and skin lift were also statistically significant and might reflect rebuilding of the collagen and elastin fiber formation in the extracellular matrix. Skin firmness improved by 24 percent, with a four-percent improvement after a single application. This was followed with a gradual improvement up to 24.7 percent at 12 weeks of product use. The clinical grading of skin lift improved by 5.4 percent after Week 4 of treatment and this effect remained stable in 43.7 percent of the subjects at Week 12.
Although the decrease in pore size from baseline was not statistically significant, 40 percent of the subjects did experience an improvement. The interpretation of fine lines and wrinkles results produced mixed outcomes in that there was a decrease in the mean percent of wrinkle depth and wrinkle volume but this effect was not statistically significant nor sustained. Despite the lack of complete resolution, more than 46 percent of the subjects experienced a reduction in the appearance of their fine lines and wrinkles. Since it takes decades to develop wrinkles, it might not be a realistic expectation to reverse this process in 12 weeks and a longer duration of treatment with a larger sample size might be required to produce statistically significant results. Other possible explanations to account for this might include an inadequate concentration of ingredients that impact this process, the resistance of unknown mechanisms of wrinkle formation to such treatment, a possible drop in treatment adherence, and the impact of selection bias on the small sample size.
Our findings confirm the effectiveness of these products in improving hydration, skin barrier function, and skin firmness. Overall, the use of a combination of constituents contained in these novel formulations support the evidence obtained from studies on individual ingredients.
The subjective benefits reported by the subjects in this study are supported by the data generated using objective instrumental measures. However, more objective evidence is needed to confirm our results, with an emphasis placed on determining optimal formulations and concentrations of natural ingredients as well as product safety and efficacy over a longer duration of treatment.
Some of these results might be due to effective blockage of inflammatory pathways, improved genomic stability, improved resilience of specialized skin cells, stabilization of the extracellular matrix, replenishment of vital nutrients, downregulation of tyrosine kinase, and other effects.
Although damage might be inflicted by inflammatory cascades, the blockage of multiple mechanisms at different points might be required to control aging, as their inter-relationships are yet to be identified. Skin aging can be modified by neutralizing excess free radicals that activate inflammatory pathways that degrade elastic fibers and have an adverse effect on the extracellular matrix. This activity can be prevented by antioxidants such as vitamin C and vitamin E or antioxidative enzymes such as superoxide dismutase, catalase, and glutathione. Superoxide dismutase and glutathione are universal reducing agents synthesized in the epidermis to defend against oxidative stress. Other actives, such as thioredoxin or glutaredoxin in conjunction with methionine sulfoxide reductase, can function as an “electron sink,” quenching free radicals.82,83 Carnosine has also been successfully employed as an antioxidant and antiglycating agent.4,18,31–33,75–77,84–86
There is also evidence available for inclusion of anti-inflammatories in skin formulations, since inflammation frequently accompanies oxidative stress and contributes to aging. Polyphenols contained in the test formulations are known to exert positive effects concerning skin hydration, moisture retention, and wrinkle formation, mainly due to their free radical scavenging activity and the regulation of tyrosine kinase.30–38,79–81
The supplementation of active nutrients with low-molecular-weight substrate molecules allows for easier permeation and has the capacity to both generate new keratinocytes, fibroblasts, and elastic fibers and improve their longevity, leading to a better skin appearance.52,53
Various growth factors and peptides have been found to downregulate various inflammatory pathways, enhance cellular growth, stimulate stem cells, reverse cell senescence, and facilitate cell renewal.42–47
Synthetic peptides designed to upregulate genes that produce elastic fibers, encourage angiogenesis, and facilitate cell migration while inhibiting proinflammatory cytokine and chemokine production may also exert significant effects.48–51,68–73
It is not possible to unravel the contribution of each component, as these interactions are complex, at times working through yet-unknown mechanisms. However, the obtained positive results are indicative of their safety and efficacy.
Many additional constituents included in LMFS and LSE (e.g., hydrolyzed collagen, sodium hyaluronate, Boswellia serrata extract, niacinamide, acetyl glucosamine, Centella asiatica extract, betulinic acid, Camellia sinensis (green tea) leaf extract) are not produced in the human body, decline with age, reduce oxidative stress, or have been shown to improve the production of vital structural proteins such as collagen and elastin.22–40 However, the end result from their interaction, mutual potentiation, and coordinating action of multiple constituents may be unpredictably different and remains unknown.
The overall improvements are likely due to individual or synergistic actions of multiple components in the two products working on intracellular and extracellular levels. These have already been demonstrated to individually decrease oxidative stress, inflammation, and cell survival while increasing the resilience and production of proteins, elastic elements, and generation of cell growth.53–83
The overall positive effects of a topical application of these formulations should be investigated at a longer time interval given the complexity of the pathophysiology and metabolism of the cellular and extracellular compartments and their impact on skin appearance.
Limitations. The limitations of the present study are the small sample size and the absence of a control group. The short duration of our study might have contributed to a limited outcome, especially in terms of chronic changes such as wrinkle formation. The use of these novel formulations produced significant positive objective and subjective results with regard to the common signs of skin aging and, therefore, present an attractive option to counteract the aging process.
Conclusion
This novel, topical antiaging skin care regimen demonstrated improvements in facial skin hydration, skin barrier function, and skin lift over a 12-week period in a small group of adult women, with no reported adverse events. Study participants reported an overall high degree of satisfaction. Our results suggest that this skin care regimen might be a useful treatment option for patients seeking aesthetic improvements in facial skin as they age. Larger, randomized, controlled trials with longer follow-up periods are needed to confirm our findings.
References
- Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16–29.
- Trautinger F. Mechanisms of photodamage of the skin and its functional consequences for skin ageing. Clin Exp Dermatol. 2001;26(7):573–577.
- Pinnell SR. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J Am Acad Dermatol. 2003;48(1):1–19; quiz 20–22.
- Zhang S, Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27(5):729–738.
- Viña J, Borras C, Abdelaziz KM, Garcia-Valles R, Gomez-Cabrera MC. The free radical theory of aging revisited: the cell signaling disruption theory of aging. Antioxid Redox Signal. 2013;19(8):779–787.
- Wenk J, Brenneisen P, Meewes C, et al. UV-induced oxidative stress and photoaging. Curr Probl Dermatol. 2001;29:83–94.
- Fisher GJ, Kang S, Varani J, et al. Mechanisms of photoaging and chronological skin aging. Arch Dermatol. 2002;138(11):1462–1470.
- Risom L, Møller P, Loft S. Oxidative stress-induced DNA damage by particulate air pollution. Mutat Res. 2005;592(1–2):119–137.
- Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360(9341):1233–1242.
- Menzel DB. The toxicity of air pollution in experimental animals and humans: the role of oxidative stress. Toxicol Lett. 1994; 72(1–3): 269–277.
- Pan MR, Li K, Lin SY, Hung, WC. Connecting the dots: from DNA damage and repair to aging. Int J Mol Sci. 2016;17(5):685.
- Gkogkolou, P, Böhm M. Advanced glycation end products: Key players in skin aging? Dermatoendocrinol. 2012;4(3):259–270.
- Liebel F, Kaur S, Ruvolo E, et al. Irradiation of skin with visible light induces reactive oxygen species and matrix-degrading enzymes. J Invest Dermatol. 2012;132(7):1901–1907.
- Sardy M. Role of matrix metalloproteinases in skin ageing. Connect Tissue Res. 2009;50(2):132–138.
- Quan T, Fisher GJ. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology. 2015;61(5):427–434.
- Sherratt MJ. Tissue elasticity and the ageing elastic fibre. Age (Dordr). 2009;31(4):305–325.
- Cadet J, Douki T, Ravanat JL. Oxidatively generated damage to cellular DNA by UVB and UVA radiation. Photochem Photobiol. 2015;91(1):140–155.
- Rinnerthaler M, Bischof J, Streubel MK, et al. Oxidative stress in aging human skin. Biomolecules. 2015;5(2):545–589.
- Del Rosso JQ, Levin J. The clinical relevance of maintaining the functional integrity of the stratum corneum in both healthy and disease-affected skin. J Clin Aesthet Dermatol. 2011;4(9):22–42.
- Pandel R, Poljšak B, Godic A, Dahmane R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013:93016.
- Scharffetter-Kochanek K, Wlaschek M, Brenneisen P, et al. UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biol Chem. 1997;378(11):1247–1257.
- Pinnell SR. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. J Am Acad Dermatol. 2003;48(1):1–19;quiz 20–22.
- Dunaway S, Odin R, Zhou L, et al. Natural antioxidants: multiple mechanisms to protect skin from solar radiation. Front Pharmacol. 2018;9:392.
- Boelsma E, Hendriks HF, Roza L. Nutritional skin care: health effects of micronutrients and fatty acids. Am J Clin Nutr. 2001;73(5):853–864.
- Fanian F, Mac-Mary S, Jeudy A, et al. Efficacy of micronutrient supplementation on skin aging and seasonal variation: a randomized, placebo-controlled, double-blind study. Clin Interv Aging. 2013;8:1527–1537.
- Lacroix S, Bouez C, Vidal S, et al. Supplementation with a complex of active nutrients improved dermal and epidermal characteristics in skin equivalents generated from fibroblasts from young or aged donors. Biogerontology. 2007;8(2):97–109.
- Hwa-Young Kim. The methionine sulfoxide reduction System: selenium utilization and methionine sulfoxide reductase enzymes and their functions. Antioxid Redox Signal. 2013;19(9):958–969.
- Stellavato A, Pirozzi AVA, Donato S, et al. Positive effects against UV-A induced damage and oxidative stress on an in vitro cell model using a hyaluronic acid based formulation containing amino acids, vitamins, and minerals. Biomed Res Int. 2018;2018:8481243.
- Wollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci. 2018;19(10):3059.
- Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51(5):1000–1013.
- Pullar JM, Carr AC, Vissers MCM. The roles of vitamin C in skin health. Nutrients. 20179(8):866.
- Nusgens BV, Humbert P, Rougier A, et al. Topically applied vitamin C enhances the mRNA level of collagens I and III, their processing enzymes and tissue inhibitor of matrix metalloproteinase 1 in the human dermis. J Invest Dermatol. 2001;116(6): 853–859.
- Kameyama K, Sakai C, Kondoh S, et al. Inhibitory effect of magnesiun L-ascorbyl-2-phosphate (VCPMG) on melanogenesis in vitro and in vivo. J Am Acad Dermatol. 1996;34(1):29–33.
- Masaki H. Role of antioxidants in the skin: anti-aging effects. J Dermatol Sci. 2010;58(2):85–90.
- Prasad S, Sung B, Aggarwal B. Age-associated chronic diseases require age-old medicine: role of chronic inflammation. Prev Med. 2012;54(Suppl):S29–S37.
- Togni S, Maramaldi G, Di Pierro F, Biondi M. A cosmeceutical formulation based on boswellic acids for the treatment of erythematous eczema and psoriasis. Clin Cosmet Investig Dermatol. 2014;7:321–327.
- Siemoneit U, Koeberle A, Rossi A, et al. Inhibition of microsomal prostaglandin E2 synthase-1 as a molecular basis for the anti-inflammatory actions of boswellic acids from frankincense. Br J Pharmacol. 2011;162(1):147–162.
- Fowler JF, Jr, Woolery-Lloyd H, Waldorf H, Saini R. Innovations in natural ingredients and their use in skin care. J Drugs Dermatol. 2010;9(Suppl 6):S72–S81.
- Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2018;19(1):70.
- Patzelt A, Lademann J, Richter H, et al In vivo investigations on the penetration of various oils and their influence on the skin barrier. Skin Res Technol. 2012;18(3):364-9.
- Aggarwal BB, Prasad S, Reuter S, et al. Identification of novel anti-inflammatory agents from ayurvedic medicine for prevention of chronic diseases: “reverse pharmacology” and “bedside to bench” approach. Curr Drug Targets. 2011;12(11):1595–1653.
- Fields K, Falla TJ, Rodan K, Bush L. Bioactive peptides: signaling the future. J Cosmet Dermatol. 2009;8(1):8–13.
- Zhang L, Falla TJ. Cosmeceuticals and peptides. Clin Dermatol. 2009;27(5):485–494.
- Cicero AFG, Fogacci F, Colletti A. Potential role of bioactive peptides in prevention and treatment of chronic diseases: a narrative review. Br J Pharmacol. 2017;174(11):1378–1394.
- Son DH,Yang DJ, Sun JS, et al. A novel peptide, nicotinyl–isoleucine–valine–histidine (NA–IVH), promotes antioxidant gene expression and wound healing in HaCaT cells. Mar Drugs. 2018;16(8):262.
- La Manna S, Di Natale C, Florio D, Marasco D. Peptides as therapeutic agents for inflammatory-related diseases. Int J Mol Sci. 2018;19(9):2714.
- Lupo MP, Cole AL. Cosmeceutical peptides. Dermatol Ther. 2007;20(5):343–349.
- Farwick M, Grether-Bec S, Marini A, et al. Bioactive tetrapeptide GEKG boosts extracellular matrix formation: in vitro and in vivo molecular and clinical proof. Exp Dermatol. 2011;20(7):602–604.
- Blanes-Mira C, Clemente J, Jodas G, et al. A synthetic hexapeptide (Argireline) with antiwrinkle activity. Int J Cosmet Sci. 2002;24(5):303–310.
- Apland JP, Biser JA, Adler M, et al. Peptides that mimic the carboxy-terminal domain of SNAP-25 block acetylcholine release at an Aplysia synapse. J Appl Toxicol. 1999;19(Suppl 1):S23–S26.
- Schagen SK. Topical peptide treatments with effective anti-aging results. Cosmetics. 2017;4(2):16.
- Thomas NV, Kim SK. Beneficial effects of marine algal compounds in cosmeceuticals. Mar Drugs. 2013;11(1):146–164.
- Pérez-Sánchez A, Barrajón-Catalán E, Herranz-López M, Micol V. Nutraceuticals for skin care: a comprehensive review of human clinical studies. Nutrients. 2018;10(4):403.
- Souyoul SA, Saussy KP, Lupo MP. Nutraceuticals: a review. Dermatol Ther (Heidelb). 2018;8(1):5–16.
- Zeng F, Harris RC. Epidermal growth factor, from gene organization to bedside. Semin Cell Dev Biol. 2014;28:2–11.
- Werner S. Keratinocyte growth factor: a unique player in epithelial repair processes. Cytokine Growth Factor Rev. 1998;9(2):153–165.
- Gold MH, Mehta RC, Smith SR, Grove GL, et al. Reduction in facial photodamage by a topical growth factor product. J Drugs Dermatol. 2008;7(9):864–871.
- Fitzpatrick RE, Rostan EF. Reversal of photodamage with topical growth factors: a pilot study. J Cosmet Laser Ther. 2003;5(1):25–34.
- Hussain M, Phelps R, Goldberg DJ. Clinical, histologic, and ultra-structural changes after use of human growth factor and cytokine skin cream for the treatment of skin rejuvenation. J Cosmet Laser Ther. 2008;10(2):104–109.
- Mehta RC, Fitzpatrick RE. Endogenous growth factors as cosmeceuticals. Dermatol Ther. 2007;20(5):350–359.
- 61. Lupo ML, Cohen JL, Rendon MI. Novel eye cream containing a mixture of human growth factors and cytokines for periorbital skin rejuvenation. J Drugs Dermatol. 2007;6(7):725–729.
- Zhang L, Falla TJ. Cosmeceuticals and peptides. Clin Dermatol. 2009;27(5):485–494.
- Fields K, Falla TJ, Rodan K, Bush L. Bioactive peptides: signaling the future. J Cosmet Dermatol. 2009;8(1):8–13.
- Sundaram H, Gold M, Waldorf H, et al. Pilot, multicenter, open-label evaluation of safety, tolerability and efficacy of a novel, topical multipotent growth factor formulation for the periorbital region. J Drugs Dermatol. 2015;14(12):1410–1417.
- Goldman MP, Biron J. Human growth factor and cytokine skin cream for facial skin rejuvenation as assessed by 3D in vivo optical skin imaging. J Drugs Dermatol. 2007;6(10):1018–1023.
- Rao J, Ehrlich M, Goldman MP. Facial skin rejeuvenation with a novel topical compound containing transforming growth factor b1 and vitamin C. Cosmet Dermatol. 2004;17:705–713.
- Gold MH, Goldman MP, Biron J. Efficacy of novel skin cream containing mixture of human growth factors and cytokines for skin rejuvenation. J Drugs Dermatol. 2007;6(2):197–201.
- Yanagisawa H, Davis EC. Unraveling the mechanism of elastic fiber assembly: the roles of short fibulins. Int J Biochem Cell Biol. 2010;42(7):1084–1093.
- Oda K, Matsuoka Y, Funahashi A, Kitano H. A comprehensive pathway map of epidermal growth factor receptor signaling. Mol Syst Biol. 2005;1:2005.0010.
- Mitchell AC, Briquez PS, Hubbell JA, Cochran JR. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016;30:1–12.
- Martin KI, Glaser DA. Cosmeceuticals: the new medicine of beauty. Mo Med. 2011;108(1):60–63.
- Llopis-Hernández V, Cantini M, González-García C. et al. Material-driven fibronectin assembly for high-efficiency presentation of growth factors. Sci Adv. 2016;2(8):e1600188.
- Ohara H, Ichikawa S, Matsumoto H, et al. Collagen-derived dipeptide, proline-hydroxyproline, stimulates cell proliferation and hyaluronic acid synthesis in cultured human dermal fibroblasts. J Dermatol. 2010;37(4):330–338.
- Atiba A, Nishimura M, Kakinuma S, et al. Aloe vera oral administration accelerates acute radiation-delayed wound healing by stimulating transforming growth factor-beta and fibroblast growth factor production. Am J Surg. 2011;201(6):809–818.
- Narda M, Peno-Mazzarino L, Krutmann J, et al. Novel facial cream containing carnosine formation of advanced glycation end-products in human skin. Skin Pharmacol Physiol. 2018; 31(6):324–331.
- Guaitolini E, Cavezzi A, Cocchi S, et al. Randomized, placebo-controlled study of a nutraceutical based on hyaluronic acid, L-carnosine, and methylsulfonylmethane in facial skin aesthetics and well-being. J Clin Aesthet Dermatol. 2019;12(4):40–45.
- Reddy VP, Garrett MR, Perry G, Smith MA. Carnosine: a versatile antioxidant and antiglycating agent. Sci Aging Knowledge Eviron. 2005;2005(18):pe12.
- Kim E, Hwang K, Lee J, et al. Skin protective effect of epigallocatechin gallate. Int J Mol Sci. 2018;19(1):173.
- Nichols, JA, Katiyar, SK. Skin photoprotection by natural polyphenols: anti-inflammatory, anti-oxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302(2):71.
- Hussain T, Tan B, Yin Y, et al. Oxidative stress and inflammation: what polyphenols can do for us?. Oxid Med Cell Longev. 2016;2016:7432797.
- Saric S, Sivamani RK. Polyphenols and sunburn. Int J Mol Sci. 2016;17(9):1521.
- Matsuo Y, Yodoi J. Extracellular thioredoxin: a therapeutic tool to combat inflammation. Cytokine Growth Factor Rev. 2013;24(4):345–353.
- Hanschmann EM, Godoy JR, Berndt C, et al. Thioredoxins, glutaredoxins, and peroxiredoxins—molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxid Redox Signal. 2013;19(13):1539–1605.
- Younus H. Therapeutic potentials of superoxide dismutase. Int J Health Sci (Qassim). 2018;12(3):88–93.
- Wagener FA, Carels CE, Lundvig DM. Targeting the redox balance in inflammatory skin conditions. Int J Mol Sci. 2013;14(5):9126–9167.
- Anja Knott A, Achterberg V, Smuda H, et al. Topical treatment with coenzyme Q10-containing formulas improves skin’s Q10 level and provides antioxidative effects. Biofactors. 2015;41(6):383–390.
- Leveque JL, Garson JC, de Rigal J. Transepidermal water loss from dry and normal skin. J Soc Cosmet Chem. 1979;30:333–343.
- Jemac GB, Serup J. Epidermal hydration and skin mechanics. Acta Derm Venereol. 1990;70(3):245–250.
- Grove GL, Grove MJ, Zerweck C, Pierce E. Comparative metrology of the evaporimeter and DermaLab TEWL probe. Skin Research & Tech. 1999;5(1):1–8.
- Ohshima H, Kinoshita S, Oyobikawa M, et al. Use of Cutometer area parameters in evaluating age-related changes in the skin elasticity of the cheek. Skin Res & Tech. 2013;19(1):e238–e242.
- Undine B, Eisner P. Hardware and measuring principle: the Cutometer. In: Elsner P, Berardesca E, Wilhelm KP. Bioengineering of the Skin: Skin Biomechanics. Abingdon, UK: Routledge; 2002: 91–98.
- Agache P, Varchon D. Skin mechanical function. In: Humbert P, Fanian F, Maibach HI, Agache P Agache’s Measuring the Skin: Non-invasive Investigations, Physiology, Normal Constants. New York, NY: Springer; 2004: 429–467.
- Lee KC, Dretzke, J, Grover, L, et al. A systematic review of objective burn scar measurements. Burns Trauma. 2016;4:14.

