Solviva® Biomaterials for Implantable Medical Devices
With over 30 years of experience supplying high performance polymers to the healthcare industry, Syensqo has become a leading provider of biomaterials for implantable medical devices. Syensqo formulates Solviva® Biomaterials, a family of specialty polymers, to meet the industry demand for metal alternatives that provide optimal performance and meet the requirements for prolonged or permanent exposure to bodily fluids and tissue in the human body. This exceptional portfolio provides safe and reliable materials for a number of implant fields including orthopedics, cardiovascular, spine, sports medicine, extremities, trauma and more. It includes:
Eviva® PSU (polysulfone) is a transparent polymer that offers toughness and strong biocompatibility. This sulfone polymer maintains its dimensional tolerance right out of the mold and requires no machining. It is a rigid thermoplastic that doesn’t absorb fluids and is artifact-free for effective MRI imaging. It is also available in opaque white.
Veriva® PPSU (polyphenylsulfone) provides unsurpassed toughness combined with transparency and excellent biocompatibility.
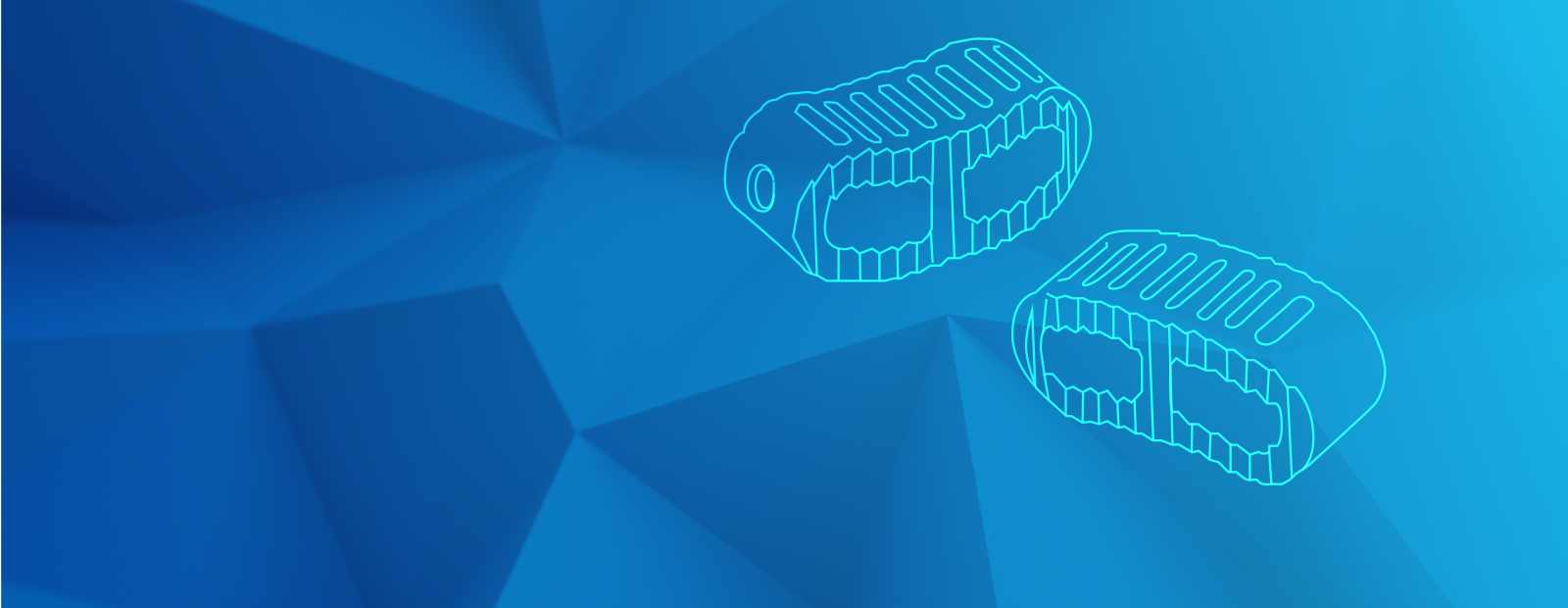
Zeniva® PEEK (polyetheretherketone) is one of the most biostable plastics available with high strength and stiffness plus excellent toughness and fatigue resistance. Zeniva® PEEK meets the requirements of ASTM F2026-07 as a polyetheretherketone (PEEK) polymer for surgical implant applications. PEEK boasts a modulus of elasticity that is similar to that of cortical bone, minimizing reduction in bone density by maintaining normal stress on surrounding bone tissue. It offers excellent biocompatibility and fatigue resistance. Zeniva® PEEK also eliminates the risk of allergic reactions to heavy metals, and its radiolucent properties will not interfere with X-ray and computed tomography scanning procedures.
Comparison of stiffness-to-weight ratios
Tensile modulus/density
All Solviva® Biomaterials can be sterilized using conventional methods, such as gamma radiation, ethylene oxide and steam. They demonstrate no evidence of cytotoxicity, sensitization, intracutaneous reactivity or acute systemic toxicity, based on biocompatibility testing as defined by ISO 10993:1. These sterilizable products are available in grades for injection molding or extrusion, as well as stock shapes for machined components.
Solviva® Biomaterials sterilization compatibility and material availability
Syensqo's specialty polymers for implantable devices* meet and exceed the highest industry standards of safety and quality excellence that OEMs require from their partners. The entire line of Solviva® Biomaterials is manufactured in our ISO 13485 and cGMP compliant dedicated facility in Georgia, USA. Materials are tested in our ISO 17025 labs and offer: Hundreds of regulatory approvals in USA, Europe, China and other regions • Available in pellets, rod stock and plate • Filled, high flow and low flow grades • Product traceability and change notification • Direct shipping and billing to processors • Validated process.
*Only products designated as part of the Solviva® family of biomaterials may be considered as candidates for medical applications implanted in the human body and devices that are in contact with bodily fluids or tissues for greater than 24 hours.
Shaping the Future of Implantables
Outstanding Structural Properties
As part of the Solviva® portfolio of specialty thermoplastics for implantable devices, these materials meet the highest standards for strength and durability. For instance, Zeniva® PEEK, used in a wide range of cages and spacers for anterior and posterior fusions, offers outstanding strength and extreme toughness. The load-bearing reliability of this medical grade PEEK makes it an excellent material for structural fixations and long-term attachment devices as it outperforms metal with its stiffness-to-weight ratios. Additionally, Veriva® PPSU (polyphenylsulfone) provides superb toughness and strength in applications such as valves and shunts which treat hydrocephalus.
Dimensional Stability
The dimensional integrity of both Eviva® PSU (polysulfone) and Veriva® PPSU offer superior moldability and manipulation into tight and complex shapes for an array of cardiovascular, neurovascular and dental applications. The flexibility of Eviva® PSU requires no expensive machining, which gives OEMs the ability to manufacture more cost-effective components for vascular and injection access ports compared to other commercial materials. Additionally, Veriva® PPSU offers exceptional moldability and is easily manipulated for optimized production of wire and lead coatings.
Impermeability and Chemical Resistance
Structural implantable devices can often come in contact with bodily tissue and fluids as well as a variety of medical substances. To endure the demanding conditions and chemical exposure that implantable medical devices face, Syensqo specialty polymers for implantable devices are designed with impermeability and chemically resistant properties. Eviva® PSU is impermeable, which makes it ideal for injection access ports. Additionally, Veriva® PPSU offers outstanding chemical resistance, which is optimal for wire and lead coatings in valves and shunts that help to regulate flow rates of essential pressure-based fluid delivery.
Optimal Radiolucency and Transparency
Syensqo’s high-performing polymers are equipped with advantageous radiolucency and transparency qualities. Zeniva® PEEK provides implantable devices with radiolucent components to provide exceptional visibility for X-rays, CT scans, MRIs and other imaging methods. The transparent qualities of Eviva® PSU and Veriva® PPSU also allow un-obstructed imaging, which surpasses the preceding capabilities of lower performing materials.
Specialty Polymers at work