Behavior - Landscape Interactions May Create Super-Spreader Environments: Vigilance-Olfactory Interactions Across Land Type and Disease Transmission Potential in the Banded Mongoose
- 1Department of Fish and Wildlife Conservation, Virginia Tech, Blacksburg, VA, United States
- 2Chobe Research Institute, Centre for Conservation of African Resources: Animals, Communities, and Land Use (CARACAL), Kasane, Botswana
A complex suite of drivers can influence infectious disease transmission with behavior and landscape spatial dynamics contributing importantly to epidemic patterns across host–pathogen-environmental systems. However, our understanding of the interaction between landscape and host behavior and its influence on spatial variability in pathogen transmission is limited. In the banded mongoose (Mungos mungo), a novel tuberculosis pathogen, Mycobacterium mungi, has emerged in Northern Botswana, which is transmitted through olfactory communication behaviors. We evaluated how associations between landscape type and mongoose behaviors affect the frequency of olfactory communication behaviors and pathogen transmission potential. We used remote sensing camera traps at den sites to eliminate observer influence across human-modified and natural landscapes (n = 18 troops, 18,229 detections of banded mongooses from 7,497 photographs). Using generalized linear mixed models, we identified a significant effect of vigilance and the interactions between vigilance and landscape, and vigilance and troop count on the frequency of olfactory behaviors. Troop count-vigilance interactions had a negative influence on olfactory communication. Vigilance, however, appeared to have a bidirectional association with olfactory communication depending on land type. In lodge areas, vigilance was associated with increased olfactory behaviors, but in landscapes with expected increases in predation risk (i.e., national park and urban land-use areas), vigilance had a negative association with olfactory behaviors. The interaction between behavior and landscape type may have the potential to create “super-spreading” environments, or transmission hotspots, where behavior-landscape interactions increase pathogen shedding and transmission potential.
Introduction
Human-mediated landscape change is increasingly linked to zoonotic disease emergence. However, outbreaks of disease have occurred across a wide array of landscape types and host–pathogen systems, challenging our ability to develop a full mechanistic understanding of the process (Brearley et al., 2012). Landscape heterogeneity can play an important role in pathogen transmission and persistence potential, creating disease hotspots or “super-spreading” habitat patches across a landscape (Paull et al., 2012). In these instances, certain landscape attributes may influence host–pathogen interactions, facilitating higher levels of pathogen shedding, exposure, and/or transmission. Understanding comparative host behavioral dynamics across landscape or habitat type is central to determining how these divergent landscapes or habitat patches influence pathogen transmission potential. This is particularly important in transforming landscapes where human population expansion exerts important influences on the biology of hosts, pathogens, and vectors (Bradley and Altizer, 2007; Magle et al., 2012; Ramalho and Hobbs, 2012; Gottdenker et al., 2014). Numerous mammalian species have successfully inhabited human-dominated ecosystems, but limited attention has been directed at understanding how these heterogenous landscapes variably influence key social behaviors such as those used in communication (Hutton and McGraw, 2016). We know even less about the manner in which changes in communication across landscape type changes infectious disease transmission, either directly (i.e., exposure and transmission) or indirectly (e.g., change in host density or contact rates).
Communication is a central feature of individual and group fitness, influenced strongly by the environment (Hutton and McGraw, 2016). A common mode of communication is through olfaction, where individuals deposit scents with chemical cues in the environment to communicate with conspecifics in various social contexts (Arakawa et al., 2008). Olfactory communication is common among mammalian species and plays a crucial role in spatial organization and sexual and other social behaviors (Stoddart, 1976; Brown and Macdonald, 1985). Information such as the depositor’s identity (Boogert et al., 2006), reproductive status (Washabaugh and Snowdon, 1998), competitive abilities (Rich and Hurst, 1999), and even health status (Kavaliers and Colwell, 1995; Penn et al., 1998; Klein et al., 1999; Willis and Poulin, 2000; Zala et al., 2004) can be communicated to conspecifics. Olfactory communication behavior can also be a direct conduit for infectious disease transmission when olfactory secretions (feces, urine, and scent marks) contain pathogens (Alexander et al., 2016). For example, banded mongooses in Northern Botswana, are infected with Mycobacterium mungi, a novel Mycobacterium tuberculosis complex pathogen. Environmental transmission occurs through communication behaviors where interactions with infected olfactory secretions (i.e., anal gland scent marks and urine) provide primary exposure and transmission of the pathogen between infected and susceptible conspecifics within and between social groups (Alexander et al., 2016). Similarly, bank voles (Myodes glareolus) transmit Puumala hantavirus through urine, a secretion used in olfactory communication (Hughes et al., 2014; Beauchamp, 2017). Latrine sites often have feces and other olfactory secretions, and in racoon (Procyon lotor), these sites have been implicated in the transmission of the raccoon roundworm [B. procyonis, (Hirsch et al., 2014)]. Understanding the drivers of olfactory communication is fundamental to understanding and predicting pathogen transmission in these host–pathogen systems, yet we know little about the factors that influences these dynamics.
Vigilance is a behavior that might be expected to influence olfactory communication. Fundamental to fitness, vigilance influences a species’ response to a suite of environmental cues such as a danger from a predator or the presence of a competitor (Beauchamp, 2015). Individuals must assess the risk in a particular environment and decide whether to invest in anti-predator behaviors (e.g., fleeing) or other fitness promoting activities [e.g., foraging, acquiring a mate, engaging in olfactory communication (Lima and Dill, 1990)]. In territorial group-living animals, vigilance can also be used to alert the group to threats arising from conspecifics and the need for territorial defense (Beauchamp, 2015). In social species, group size can also influence the frequency of vigilance behaviors (Roberts, 1996). Olfactory signals themselves can elicit the occurrence of vigilance behaviors within and between species (Wikenros et al., 2017). The importance of land use on olfactory communication behaviors has also been observed across various mammalian species [e.g., wolves (Canis lupus) and lynx (Lynx lynx) (Zub et al., 2003; Krofel et al., 2017) with even finer level habitat preferences observed in scent marking behaviors (i.e., deer, (Miller et al., 1987)]. For seasonal breeders, reproductive activities and the presence of pups may also influence scent marking and vigilance behaviors [e.g., banded mongoose, meerkats, and yellow mongoose (Gilchrist et al., 2008; Le Roux et al., 2008; Müller and Manser, 2008; Jordan et al., 2010, 2011b; Jojola, 2011; Cant et al., 2013)].
Using our banded mongoose–M. mungi pathogen system in Northern Botswana, we evaluated the spatial patterns of olfactory communication in relation to vigilance, season, group size, land use, and habitat. Banded mongooses in our long-term study site live across diverse land-use areas from urban centers to protected landscapes. Olfactory behaviors play a central role in inter- and intra-group communication (Jordan et al., 2010), territorial defense (Rood, 1975; Müller and Manser, 2007), reproduction (Jordan et al., 2010, 2011a,b), and pathogen transmission of M. mungi (Alexander et al., 2016). We tested the a priori prediction that vigilance behaviors differ by landscape type, divergently influencing the frequency of olfactory communication and spatial variation in pathogen transmission potential.
Materials and Methods
Study Site and Species
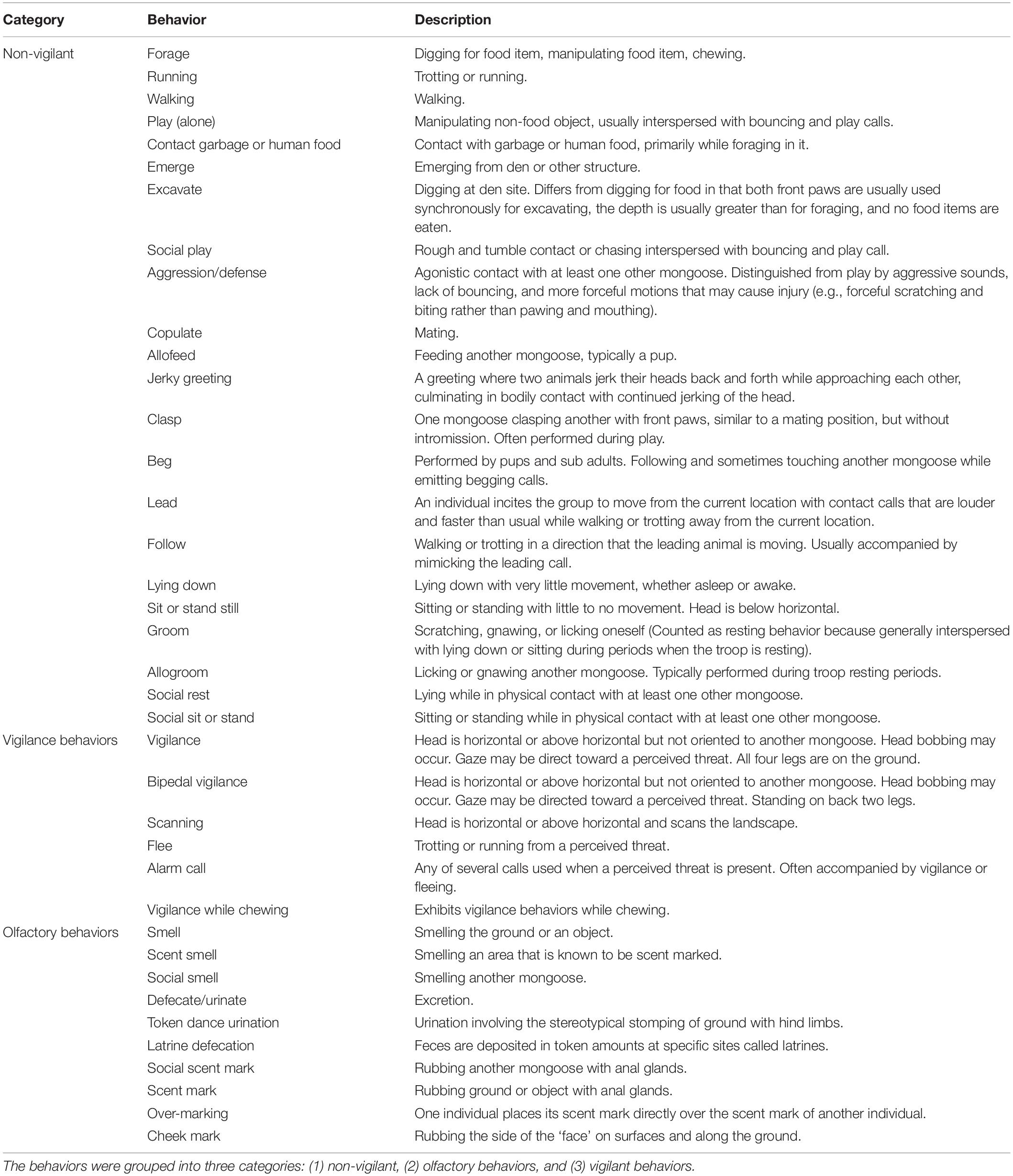
In Northern Botswana, study troops occurred across land-use type, which included the urban town of Kasane, the urban-transiting village of Kazungula, undeveloped land areas, and the protected Chobe National Park (Figure 1). Troops occurred along the riparian areas near Baikiaea plurijuga-dominated woodlands. Botswana has a wet (November–March) and dry season (April–October).
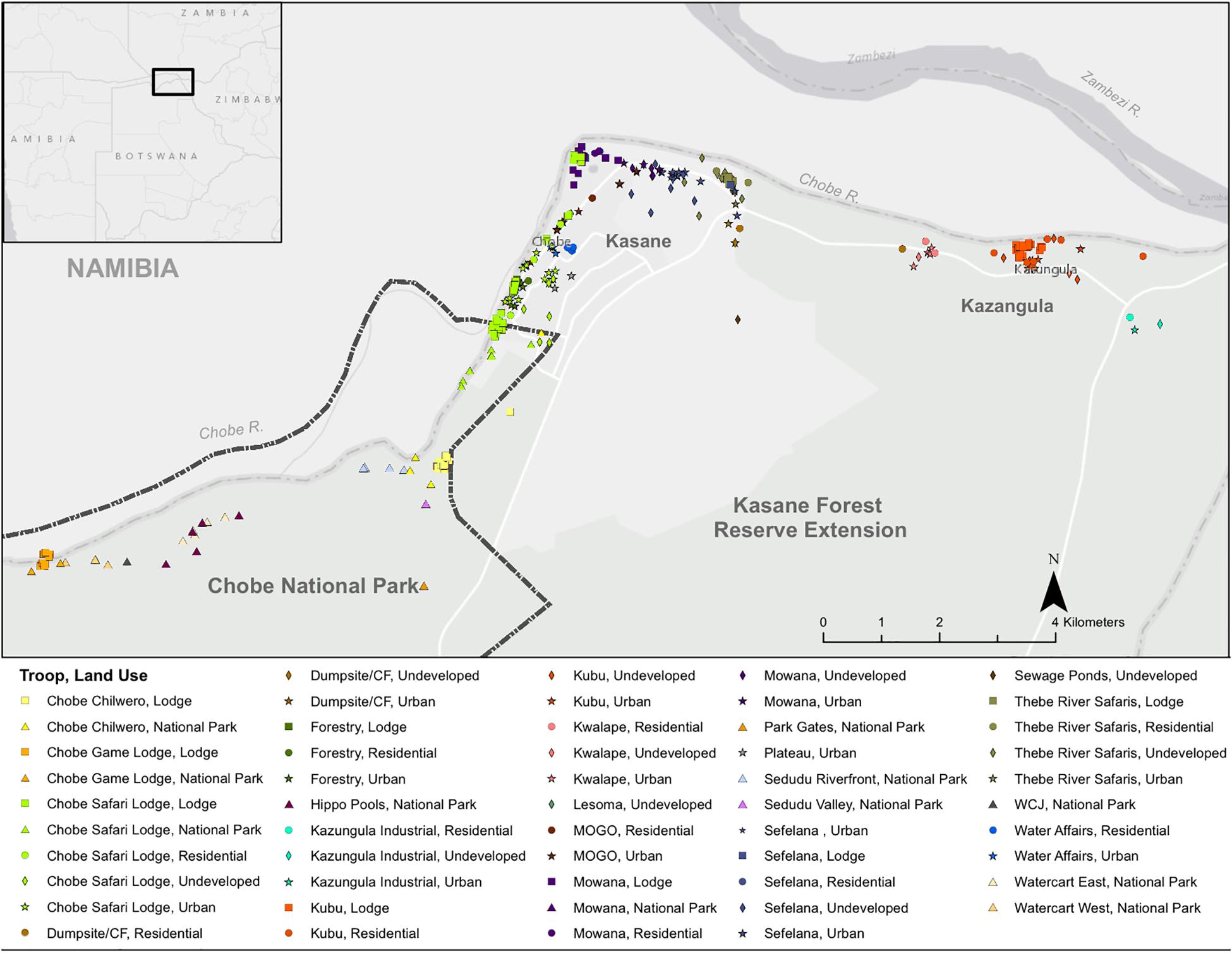
Figure 1. Study Site. The study was conducted in Northern Botswana. Banded mongoose troops occurred along the Chobe River in the riparian areas backed by the Chobe Forest Reserve (dark green boundary and stipple) and Chobe National Park (light green). Banded mongoose den sites are colored by the troop (n = 15) that used the site and the symbol provides the land type where the den was located. Lodge sites occurred within both the protected and unprotected landscapes (solid squares).
Banded mongooses are small (<2 kg), diurnal mesocarnivores that live in social groups or troops ranging from five to 65 individuals (Rood, 1975). Mongooses are territorial and occupy home ranges that spatially decrease with increasing association with humans (Laver and Alexander, 2018). Home ranges are defended against intrusions and boundaries can be demarcated by feces, urine, and other scent marks (Rood, 1975; Müller and Manser, 2007). Intertroop conflicts occur and can result in mortality, but they can also lead to intergroup group breeding events and/or movement of individuals between groups (Jordan et al., 2010).
Banded mongooses experience predation from a number of species including birds of prey, particularly martial eagles (Polemaetus bellicosus) (Rood, 1983), marabou storks (Leptoptilos crumenifer) (Otali and Gilchrist, 2004), African lions (Panthera leo) (Rood, 1975), African rock pythons (Python sebae) (Rood, 1975; Otali and Gilchrist, 2004; Laver et al., 2012), warthogs (Phacochoerus africanus), and monitor lizards (Varanus exanthematicus) (Otali and Gilchrist, 2004). Banded mongoose troop members cooperatively gather to inspect predator cues (Müller and Manser, 2007; Furrer and Manser, 2009) and bunch together to attack or mob predators and competitors (Rood, 1975). They also emit alarm calls, where pack members rush to assist individuals calling in distress (Furrer and Manser, 2009). In the human-dominated landscape, mortality is more commonly associated with vehicles, human conflict, and predation by domestic animals than in other areas (Laver and Alexander, 2018). Human responses to banded mongooses can be quite divergent, from feeding mongooses to direct persecution when banded mongooses are perceived as pests.
In Northern Botswana, banded mongoose study troops occur across different land-use types, including the urban town of Kasane, the urban-transitioning village of Kazungula, undeveloped land areas, and the protected Chobe National Park (Figure 1). Troops primarily inhabit riparian areas near B. plurijuga-dominated woodlands.
Mycobacterium mungi-infected mongooses are identifiable through observation of clinical symptoms of infection including anorexia, hunched body posture, matted fur, excessive watering of the eye, sneezing, nasal enlargement and/or deviation of the nasal planum accompanied with a build-up of mucus, enlarged testicles, and lethargy. Sick individuals are not evicted from the troop, but they often lag behind during group movements (Alexander et al., 2016).
Behavioral Data Collection
Radio Tracking and Camera Traps
Fifteen study banded mongoose troops were followed with the use of radio telemetry. Within each troop, one or two banded mongooses were trapped and fitted with radio collars as previously described (Alexander et al., 2010). Collared troops were tracked 5 days a week, alternating seven troops on the first day and the other eight troops the following day.
A challenging problem in the study of wildlife behavior is obtaining observational data that is not influenced by the observer. Banded mongoose troops range across land type and respond strongly to the observation of humans in certain land types (i.e., lodge, urban, residential, and national park) or in places they do not expect to see people (e.g., behind certain buildings or in hedgerows). Fleeing responses are common, making the collection of unbiased behavior data challenging. To overcome these limitations, we deployed camera traps at den sites, allowing us to collect behavioral data without observer influence. This also allowed us to capture data across troops and land-use in specific study locations (i.e., dens) that would be common to all study troops across land type. The den sites used by the mongoose were the unit of observation for this study. Each den site was characterized by land-use category: (1) lodge areas (commercial hotel and their grounds, including landscaped areas that were watered daily, guest rooms, kitchens, and staff housing), (2) residential areas, including private homes and local village residential plots, (3) urban areas, such as the hospital and shopping areas, (4) undeveloped areas not within a protected area, but still susceptible to some human or vehicle traffic, and (5) the national park, which was the protected area of Chobe National Park. At each den site the dominate habitat was identified [Capparis tomentosa shrub, gallery forest (riparian), Chobe River sandbank, degraded riparian forest, shrub savanna, and teak woodland] as previously described (Nichols and Alexander, 2019). Troop count data were visually obtained through radio tracking activities and the troop number was assumed to be constant from the last count obtained where the troop number could not be obtained for a particular den night.
We placed cameras at study den sites from January 2016 to March 2017. As banded mongooses change dens every 2–3 days, remote sensing cameras were placed at the identified den sites of the troops tracked that day and were moved when the troop vacated the site. If the new den was not located during the day, we used homing telemetry to track the troop and locate mongooses at night while the troop was in the den. If the den was within a gated area, or an area considered dangerous to enter at night, we returned the next morning and attempted to locate the den and place the camera before banded mongooses emerged from the den. In the event that the animals were out of the den before the camera could be placed, the camera was deployed later in the day, with data collection commencing at the next den emergence event. Cameras were placed using naturally occurring objects (e.g., trees, shrubs, and fences), as well as self-made mounts to obtain a favorable angle and distance between the camera and the area of interest.
Animal research activities were conducted through the use of an approved protocol from the Virginia Tech Institutional Animal Care and Use Committee (IACUC #16-217) and under permit from the Botswana Ministry of Environment, Natural Resources Conservation and Tourism (EWT 8/36/4 XXVI).
Behavioral Observations From Photographs
For each photograph, the date and time, number of mongooses within each photograph, and their behavioral state (i.e., olfactory, marking, non-vigilant, and vigilant) were tallied (see Table 1 for ethogram). Both marking and olfactory behaviors contributed to olfactory communication. Marking behaviors included: (1) anal scent marks, which are deposited by dragging secretions from the anal gland across a surface, (2) cheek marks or wipes, (3) urination or defecation, (4) urination followed by stomping the back limbs, coined “token dance urination,” and (5) feces that are deposited at specific marking sites called latrines (Müller and Manser, 2007; Jordan et al., 2010; Fairbanks et al., 2014). Olfaction behaviors crucial for investigating chemical cues included: (1) smelling the ground or an object, (2) smelling the location where a scent was deposited, and (3) smelling another mongoose. All other behaviors were grouped as being either vigilant and non-vigilant in nature (Table 1).
Observation Time
The length of camera observation periods varied by trap event and den sites. Each camera trap event was defined as the start time in which the first photo was taken to the end time of the series. If there were no photos for 10 min, the next photo would be considered a new event (Flint et al., 2016). When a single photograph was collected, it was given the lowest time interval designation of 10 s, since that is the lapse time for the remote sensing camera traps to capture another photograph.
Data Analysis
We undertook all statistical analyses in the R program, version 3.6.1 (R Core Team, 2013). We evaluated the independent variables for potential collinearity with the ggpairs function in the R package GGaly. To evaluate the possibility of temporal collinearity, a new sampling event was delineated when there was a cessation of photos captured by the camera traps for a period ≥10 min (see section “Observation Time”). We evaluated the data for zero inflation using the zeroinfl and Vuong functions in the R package pscl where the zero-inflated model is compared to the non-zero-inflated analog using the Vuong z statistic. We also used the testzeroinflation function in the DHARMa package. We evaluated the relationship between land use and olfactory communication and land use and vigilance using a pairwise Wilcox test with Bonferroni adjusted p-values for multiple comparisons.
We used generalized linear mixed effects models (glmm) to analyze variables influencing olfactory communication. Models were constructed with the glmmTMB package using the negative binomial response distribution [nbinom2 parameterization with variance = μ(1 + μ/k, link = log)]. glmms are an extension of linear mixed models, providing a framework for analyzing data collected according to some type of grouping rather than that obtained from independent observations. They provide the ability to include fixed and random predictor variables and have the ability to handle uneven sampling as well as non-normal data types. In our glmm, the dependent variable was the frequency of olfactory communication behaviors. The den sites and troop were used as the random effects [1| Den + (1| Troop)], controlling for non-independence among data points by den and troop. The fixed effects were set as land-use, habitat, troop count, season (wet and dry), and vigilance. As noted earlier, we hypothesized a priori that olfactory behaviors would be influenced by vigilance according to land type; therefore, we included an interaction between vigilance and land use. Based on the literature, we also included an interaction between vigilance and troop count.
The number of mongooses in the photo, observation minutes, and photographs obtained during an observation event varied between and within den sites. To standardize data and account for the unequal observations, the offset was calculated by the equation
where p is the number of photos, t is the observation time in minutes for that observational period, and b is the number of banded mongooses observed across all photos in that observational period. We used a multi-model inference approach with a backward method of variable selection using the Akaike’s Information Criterion (AIC) (Burnham and Anderson, 2003). The DHARMa package was used to evaluate model fit and provides specifically for glmm model structures and the glmmTMB output (Hartig, 2019). We used the car package and ANOVA function to compare glmmTMB model fixed effects using the Wald χ2 statistic (Type II). We used the AIC model weight to select the top model when ΔAICc < 2. The AIC model weight is considered to be the conditional probability for each model in the model set (Wagenmakers and Farrell, 2004).
Results
From January 2016 to March 2017, we sampled 281 den nights from 18 troops with a mean troop size of 28 individuals (SD 7.04, range 8–50, median 26). There were 1,815 observation periods obtained from 15 radio-collared troops and three opportunistically sampled troops with a total of 18,229 detections of banded mongooses from 7,497 photographs. Across events, mean vigilance (2.03, SD 7.5 range 0–190), olfactory (0.47, SD 1.33, range 0–15), and non-vigilant behaviors (7.55, SD 23, range 0–510) were assessed. Non-vigilant behavioral data collected in this study are reported for methodological reasons but are not used in any further analyses.
Olfactory Communication Behavior by Land Use Type
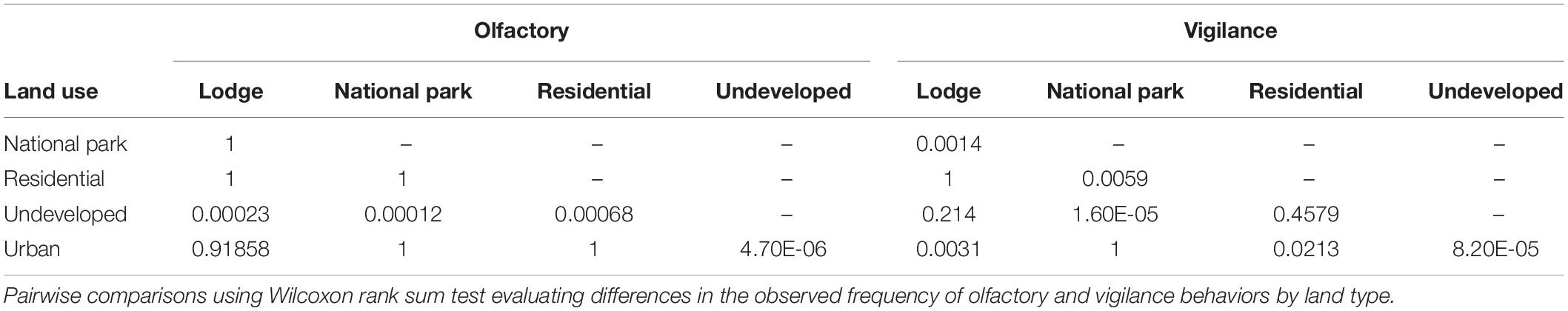
The frequency of olfactory and vigilance behaviors by land-use type are provided in Figure 2. The frequency of olfactory behaviors was lower in the undeveloped land type compared to all other land categories (p ≤ 0.0006; Table 2). Differences in vigilance behaviors by land type appeared more complex, with significant differences noted between various land classes. In contrast to that observed for olfactory behaviors, the frequency of vigilance behaviors in the undeveloped land area differed significantly only in the National Park land type (p ≤ 0.0001).
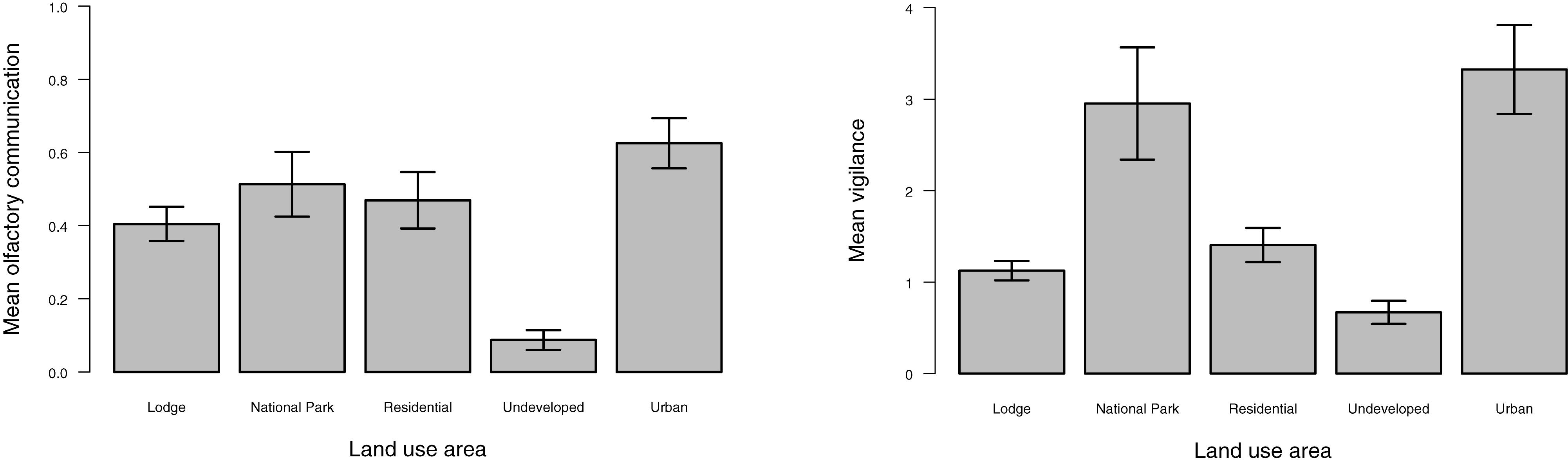
Figure 2. Mean frequency of olfactory and vigilance behaviors observed by land use. Bars represent the standard error.
Model Selection
Zero inflation in the dataset could not be detected and was, therefore, not included in the model structure. Misspecification of predictor variables could not be detected on evaluation of the DHARMa scaled residuals or with the quantile–quantile (q–q) plot. While habitat and season were initially included in our comprehensive model, there was no support for their retention.
In our top model (AICwi = 0.68, Table 3), vigilance and the interaction of vigilance with land use and vigilance with troop count explained the variation in olfactory communication behavior across den sites and troops (p < 0.05, fixed affects Wald Test Chi square Type II, Table 4). The next competing model (ΔAICc < 2, wi = 0.38) was similar in construction but without inclusion of troop count as an interacting variable. Vigilance behavior at the lodges had a significantly positive relationship with olfactory communication but a negative association in the urban and national park land areas. Vigilance interacted with troop count to negatively (−0.012) influence the frequency of olfactory communication behaviors (t = −4.59, p = 0.04, Table 5).
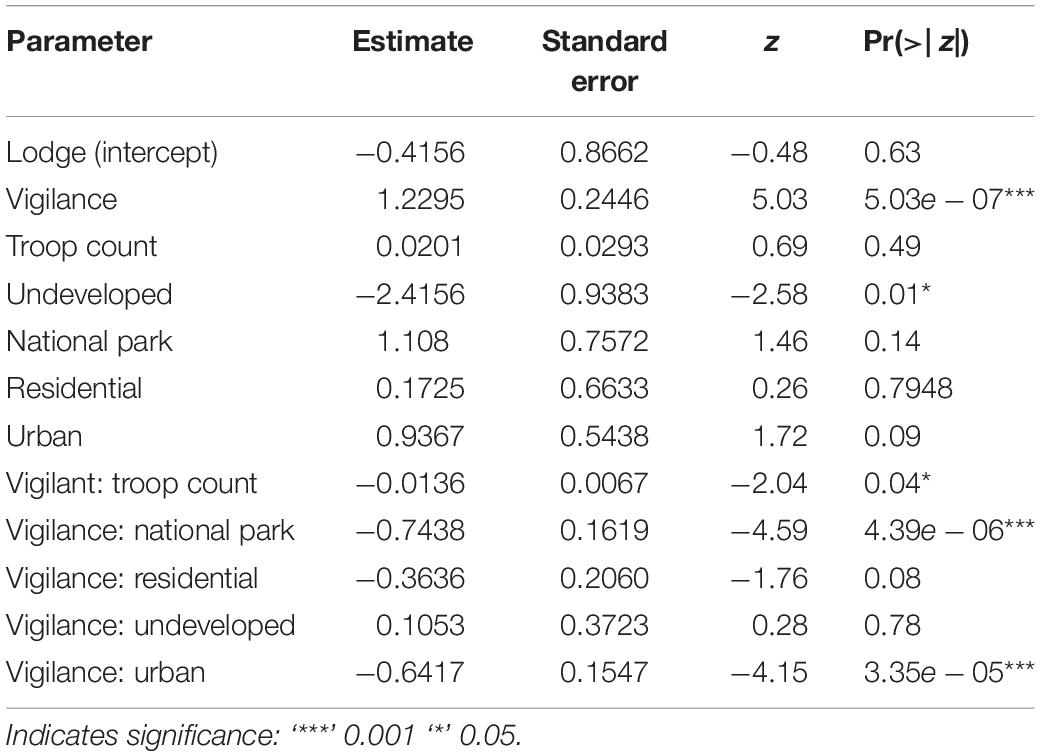
Table 5. Parameter estimates of our best supported glmm model (based on AIC wi) for banded mongoose olfactory communication in Northern Botswana.
Discussion
Interactions between behavior and land-use type can introduce important non-linearities into infectious disease transmission dynamics, particularly for pathogens transmitted through environmental pathways. Unpacking these relationships can be challenging, as is our ability to model these complex systems. In this study, glmm model results suggest that complex interactions occur between behavior and heterogeneous landscapes, influencing pathogen transmission potential in a bidirectional manner according to land class. Group size may also interact with vigilance to influence these dynamics.
Mean olfactory communication was the highest in the urban and national park land types and the lowest in the undeveloped area (Figure 2), with this latter land type differing from all other land areas (Table 2). Mean vigilance varied significantly by certain land types (Table 2) with the lowest mean levels in the undeveloped land class and the highest in the urban and national park land areas (Figure 2).
Our top model suggests that vigilance interacts with land use and is associated with decreased olfactory communication behavior in certain land types (e.g., national park and urban) and increases in other land types (tourism lodges, Table 3). This can have an important and divergent influence on the spatial occurrence of olfactory communication across land type and, consequently, pathogen shedding and transmission potential. On average, at lodge land areas where tourism facilities occur, every additional act of vigilance was associated with a 1.23 increase in olfactory communication (t = 5.03, p = << 0.0001, Table 5; Vigilance, referencing lodge land-use areas as the intercept). That is, the more vigilance behaviors that were observed, the more mongooses in this land-use type engaged in olfactory communication behaviors. The lodge land type supports tourism and wildlife viewing opportunities and humans (tourists and local employees) in these land areas are carefully controlled to promote the occurrence of wildlife in these land types. Predation risk appears to be reduced with lodge environments providing some protection from predators, both human and animal, potentially decreasing predator avoidance behaviors (e.g., fleeing) that would be incompatible with olfactory communication. In addition, lodges typically have fences to deter megafauna from entering the premises, but other non-predatory animals, such as warthogs (Phacochoerus africanus), vervet monkeys (Chlorocebus pygerythrus), and Chobe bushbuck (Tragelaphus Scriptus ornatus) commonly utilize these areas. These species may contribute to improved vigilance where heterospecific alarms are utilized by banded mongooses (Mueller and Manser, 2008). Riparian forests are often intact in this region, providing mature, closed canopies (Nichols and Alexander, 2019) that enhance protection from aerial predators. Manicured and expansive lawns without woody vegetation or buildings may also provide greater line-of-sight for predator detection. In these environments, food waste can be found across seasons in lodge garbage sites, camp sites, and kitchen facilities. Increased concentration of troops around these human-associated resources (Laver and Alexander, 2018; Nichols and Alexander, 2019) may result in more encounters with neighboring troops, where troop vigilance and territorial defense is associated with increased scent marking and inspection (Rood, 1975; Müller and Manser, 2007). Banded mongoose troops respond more aggressively to known neighboring troops than unknown troops, which is likely related to greater perceived competition for resources as well as breeding opportunities (Müller and Manser, 2007). During these inter-pack encounters, some individuals may rush toward the interaction, while others will mark or counter-mark objects in the area during these events (Cant et al., 2002; Müller and Manser, 2007).
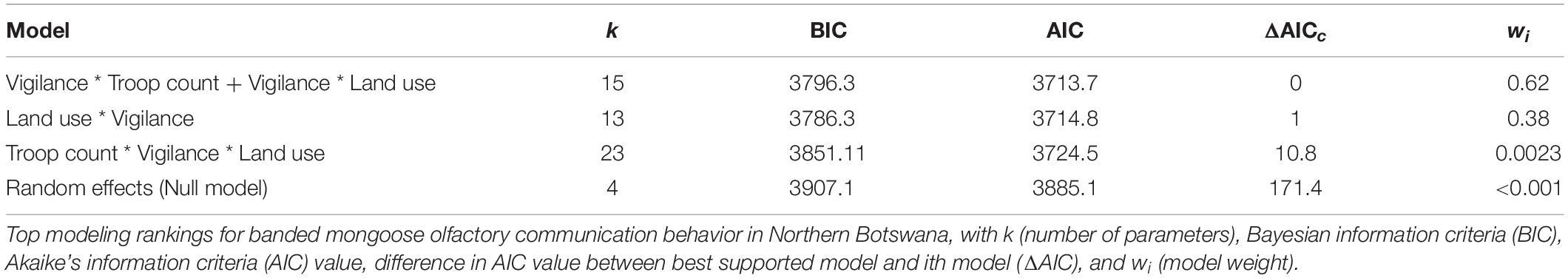
In contrast, vigilance behaviors in urban and national park land types were associated with a reduction in olfactory communication behaviors (Vigilance: National Park, −0.74, t = −4.59, p = << 0.0001 and Vigilance: Urban, −0.64, t = −4.15, p << 0.0001). Mongooses in urban environments experience high levels of mortality from vehicle collisions, humans (e.g., stoning, pouring hot oil in dens), and a suite of other avian and domestic predators (e.g., domestic dogs). When humans and/or other predators are detected in these areas, this often elicits an immediate flight response from mongooses with olfactory communication less likely to occur in conjunction with fleeing behavior (Figure 3). In urban environments, vigilance may even be heightened, as threat detection might be more difficult due to more concentrated infrastructure in these built environments and limited line-of-sight available to mongooses. Likewise, in the national park, mongooses are increasingly vulnerable to predation, with a greater diversity and density of natural predators in this land-use area (both aerial and terrestrial).

Figure 3. (A) Vigilance, (B) flight, and (C) scent marking behavior in a banded mongoose study troop.
The undeveloped land areas had a negative influence on olfactory communication (−2.5, t = −2.58, p = 0.01) and the lowest level of olfactory communication by land type (Table 2). The undeveloped land areas also had the lowest olfactory communication counts, which were significantly different from all other land types (p < 0.0005). These land types occur largely around and near human-modified landscapes (i.e., lodges, urban, and residential). In contrast to the other land types, predation (human and animal) and territorial defense needs appear to be reduced. Predators are rare due to human conflict and persecution. Human presence in these areas is also relatively rare due to a lack of infrastructure and roads and the occurrence of dangerous animals such as buffalo and elephant. Territorial defense may still be needed, however, against intrusion of conspecifics, but the lack of human refuse and anthropogenic denning resources may lower competition for these areas in comparison to the urban and lodge landscapes (Laver and Alexander, 2018).
In our model, vigilance also interacted negatively with group size to influence the frequency of olfactory communication behaviors (p = 0.04). This is consistent with other studies where a negative correlation between group size and vigilance across avian and mammalian species has been observed, also known as the “many eyes hypothesis.” Reduction in predation risk is then thought to be an important evolutionary driver for the development of grouping behaviors (Beauchamp, 2008, 2017). Other studies, however, have failed to identify an interaction, suggesting that these relationships may have more context-specific interactions influencing these dynamics (Beauchamp, 2017).
Behavior – landscape interactions appear to be an important factor determining the spatial patterns of pathogen shedding and transmission potential for M. mungi. These same dynamics may also influence the transmission of other infectious diseases that are transmitted through olfactory secretions, for example, pathogenic Leptospira sp., which is transmitted through urine. This pathogen was found in the kidneys of nearly half of the sampled mongooses in our study site (Jobbins and Alexander, 2015). Likewise, Eurasian badgers (Meles meles) are endemically infected with Mycobacterium bovis, and like mongooses, this species uses both feces and anal gland secretions in territorial defense (Roper et al., 1993). The exact mechanism of environmental transmission of M. bovis to cattle is unknown, but we speculate that a similar transmission mechanism may also occur in this species, potentially influenced by landscape in a similar manner (Alexander et al., 2016).
Conclusion
While most studies assess the trade-offs associated with vigilance and, for example, feeding behavior (Lima and Dill, 1990), little is known about the tradeoffs between vigilance and olfactory communication and how landscape may influence these dynamics. Our results identify important interactions between vigilance and land type and olfactory communication with critical implications to pathogen transmission potential. Here, complex landscapes may influence host behavior, modifying pathogen transmission dynamics across land type, potentially creating super-spreading areas, or hotspots, of environmental disease transmission. Characterization of these complex interactions is fundamental to understanding the impacts of urbanization and other drivers of landscape change and their interaction with behavior. It is also central to both forecasting and control of emerging infectious diseases that threaten both human and animal health.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The animal study was approved by the Virginia Tech Institutional Animal Care and Use Committee (IACUC #16-217).
Author Contributions
KA: conceptualization, methodology, supervision, funding acquisition, statistical analyses and write up, project administration, writing – original draft, writing –final editing, and revisions for publication. CN: field work, data collection and curation, initial statistical assessments, and writing – original draft.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This project was supported by the National Science Foundation Ecology and Evolution of Infectious Diseases (#1518663).
Acknowledgments
We are grateful to the CARACAL team for their assistance in the field and reviewing of photographs. We thank the Department of Wildlife and National Parks for allowing us to conduct this research and all the private land owners and hotel and lodge managers who were so supportive and allowed us to deploy our cameras.
References
Alexander, K. A., Laver, P. N., Michel, A. L., Williams, M., van Helden, P. D., Warren, R. M., et al. (2010). Novel Mycobacterium tuberculosis complex pathogen, M. mungi. Emerg. Infect. Dis. 16:1296.
Alexander, K. A., Sanderson, C. E., Larsen, M. H., Robbe-Austerman, S., Williams, M. C., and Palmer, M. V. (2016). Emerging tuberculosis pathogen hijacks social communication behavior in the group-living banded mongoose (Mungos mungo). mBio 7:e00281-16.
Arakawa, H., Blanchard, D. C., Arakawa, K., Dunlap, C., and Blanchard, R. J. (2008). Scent marking behavior as an odorant communication in mice. Neurosci. Biobehav. Rev. 32, 1236–1248. doi: 10.1016/j.neubiorev.2008.05.012
Beauchamp, G. (2008). What is the magnitude of the group-size effect on vigilance? Behav. Ecol. 19, 1361–1368. doi: 10.1093/beheco/arn096
Beauchamp, G. (2015). Animal Vigilance: Monitoring Competitors and Predators. Oxford: Academic Press.
Beauchamp, G. (2017). Disentangling the various mechanisms that account for the decline in vigilance with group size. Behav. Process. 136, 59–63. doi: 10.1016/j.beproc.2017.01.014
Boogert, N. J., Hofstede, F. E., and Monge, I. A. (2006). The use of food source scent marks by the stingless bee Trigona corvina (Hymenoptera: Apidae): the importance of the depositor’s identity. Apidologie 37, 366–375. doi: 10.1051/apido:2006001
Bradley, C. A., and Altizer, S. (2007). Urbanization and the ecology of wildlife diseases. Trends Ecol. Evol. 22, 95–102. doi: 10.1016/j.tree.2006.11.001
Brearley, G., McAlpine, C., Bell, S., and Bradley, A. (2012). Influence of urban edges on stress in an arboreal mammal: a case study of squirrel gliders in southeast Queensland. Australia. Landscape Ecol. 27, 1407–1419. doi: 10.1007/s10980-012-9790-8
Burnham, K. P., and Anderson, D. R. (2003). Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Berlin: Springer Science & Business Media.
Cant, M. A., Otali, E., and Mwanguhya, F. (2002). Fighting and mating between groups in a cooperatively breeding mammal, the banded mongoose. Ethology 108, 541–555. doi: 10.1046/j.1439-0310.2002.00795.x
Cant, M. A., Vitikainen, E., and Nichols, H. J. (2013). Demography and social evolution of banded mongooses. Adv. Stud. Behav. 45, 407–445. doi: 10.1016/b978-0-12-407186-5.00006-9
Fairbanks, B. M., Hawley, D. M., and Alexander, K. A. (2014). No evidence for avoidance of visibly diseased conspecifics in the highly social banded mongoose (Mungos mungo). Behav. Ecol. Sociobiol. 69, 371–381. doi: 10.1007/s00265-014-1849-x
Flint, B. F., Hawley, D. M., and Alexander, K. A. (2016). Do not feed the wildlife: associations between garbage use, aggression, and disease in banded mongooses (Mungos mungo). Ecol. Evol. 6, 5932–5939. doi: 10.1002/ece3.2343
Furrer, R. D., and Manser, M. B. (2009). Banded mongoose recruitment calls convey information about risk and not stimulus type. Anim. Behav. 78, 195–201. doi: 10.1016/j.anbehav.2009.05.002
Gilchrist, J., Otali, E., and Mwanguhya, F. (2008). Caregivers recognize and bias response towards individual young in a cooperative breeding mammal, the banded mongoose. J. Zool. 275, 41–46. doi: 10.1111/j.1469-7998.2007.00405.x
Gottdenker, N. L., Streicker, D. G., Faust, C. L., and Carroll, C. (2014). Anthropogenic land use change and infectious diseases: a review of the evidence. Ecohealth 11, 619–632. doi: 10.1007/s10393-014-0941-z
Hartig, F. (2019). DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R package.
Hirsch, B. T., Prange, S., Hauver, S. A., and Gehrt, S. D. (2014). Patterns of latrine use by raccoons (Procyon lotor) and implication for Baylisascaris procyonis transmission. J. Wildl. Dis. 50, 243–249. doi: 10.7589/2013-09-251
Hughes, N. K., Helsen, S., Tersago, K., and Leirs, H. (2014). Puumala hantavirus infection alters the odour attractiveness of its reservoir host. Oecologia 176, 955–963. doi: 10.1007/s00442-014-3072-x
Hutton, P., and McGraw, K. J. (2016). Urban Impacts on oxidative balance and animal signals. Front. Ecol. Evol. 4:54. doi: 10.3389/fevo.2016.00054
Jobbins, S. E., and Alexander, K. A. (2015). Evidence of Leptospira sp. infection among a diversity of African wildlife species: beyond the usual suspects. Trans. R. Soc. Trop. Med. Hyg. 109, 349–351. doi: 10.1093/trstmh/trv007
Jojola, S. (2011). Social Organization and Olfactory Communication in Brown Bears, Eurasian Beavers, and Yellow-Bellied Marmots. PhD thesis, Norwegian University of Life Sciences, Norway.
Jordan, N. R., Mwanguhya, F., Furrer, R. D., Kyabulima, S., Rüedi, P., and Cant, M. A. (2011a). Scent marking in wild banded mongooses: 2. Intrasexual overmarking and competition between males. Anim. Behav. 81, 43–50. doi: 10.1016/j.anbehav.2010.07.009
Jordan, N. R., Mwanguhya, F., Kyabulima, S., Ruedi, P., and Cant, M. A. (2010). Scent marking within and between groups of wild banded mongooses. J. Zool. 280, 72–83. doi: 10.1111/j.1469-7998.2009.00646.x
Jordan, N. R., Mwanguhya, F., Kyabulima, S., Rüedi, P., Hodge, S. J., and Cant, M. A. (2011b). Scent marking in wild banded mongooses: 3. Intrasexual overmarking in females. Anim. Behav. 81, 51–60. doi: 10.1016/j.anbehav.2010.10.007
Kavaliers, M., and Colwell, D. D. (1995). Discrimination by female mice between the odours of parasitized and non-parasitized males. Proc. R. Soc. Lond. B 261, 31–35. doi: 10.1098/rspb.1995.0113
Klein, S. L., Gamble, H. R., and Nelson, R. J. (1999). Trichinella spiralis infection in voles alters female odor preference but not partner preference. Behav. Ecol. Sociobiol. 45, 323–329. doi: 10.1007/s002650050567
Krofel, M., Hoèevar, L., and Allen, M. L. (2017). Does human infrastructure shape scent marking in a solitary felid? Mammal. Biol. Zeitschrift für Säugetierkunde 87, 36–39. doi: 10.1016/j.mambio.2017.05.003
Laver, P. N., and Alexander, K. A. (2018). Association with humans and seasonality interact to reverse predictions for animal space use. Mov. Ecol. 6:5. doi: 10.1186/s40462-018-0123-7
Laver, P. N., Ganswindt, A., Ganswindt, S. B., and Alexander, K. A. (2012). Non-invasive monitoring of glucocorticoid metabolites in banded mongooses (Mungos mungo) in response to physiological and biological challenges. Gen. Comp. Endocrinol. 179, 178–183. doi: 10.1016/j.ygcen.2012.08.011
Le Roux, A., Cherry, M. I., and Manser, M. B. (2008). The effects of population density and sociality on scent marking in the yellow mongoose. J. Zool. 275, 33–40. doi: 10.1111/j.1469-7998.2007.00404.x
Lima, S. L., and Dill, L. M. (1990). Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640. doi: 10.1139/z90-092
Magle, S. B., Hunt, V. M., Vernon, M., and Crooks, K. R. (2012). Urban wildlife research: past, present, and future. Biol. Conserv. 155, 23–32. doi: 10.1016/j.biocon.2012.06.018
Miller, K. V., Kammermeyer, K. E., Marchinton, R. L., and Moser, E. B. (1987). Population and habitat influences on antler rubbing by white-tailed deer. J. Wildl. Manag. 51, 62–66.
Mueller, C. A., and Manser, M. B. (2008). The information banded mongooses extract from heterospecific alarms. Anim. Behav. 75, 897–904. doi: 10.1016/j.anbehav.2007.07.012
Müller, C. A., and Manser, M. B. (2007). ‘Nasty neighbours’ rather than ‘dear enemies’ in a social carnivore. Proc. R. Soc. B 274, 959–965. doi: 10.1098/rspb.2006.0222
Müller, C. A., and Manser, M. B. (2008). Mutual recognition of pups and providers in the cooperatively breeding banded mongoose. Anim. Behav. 75, 1683–1692. doi: 10.1016/j.anbehav.2007.10.021
Nichols, C. A., and Alexander, K. A. (2019). Characteristics of banded mongoose (Mungos mungo) den sites across the human-wildlife interface in Northern Botswana. Mamm. Biol. 97, 80–87. doi: 10.1016/j.mambio.2019.03.005
Otali, E., and Gilchrist, J. S. (2004). The effects of refuse feeding on body condition, reproduction, and survival of banded mongooses. J. Mammal. 85, 491–497. doi: 10.1644/brg-021
Paull, S. H., Song, S., McClure, K. M., Sackett, L. C., Kilpatrick, A. M., and Johnson, P. T. (2012). From superspreaders to disease hotspots: linking transmission across hosts and space. Front. Ecol. Environ. 10:75–82. doi: 10.1890/110111
Penn, D., Schneider, G., White, K., Slev, P., and Potts, W. (1998). Influenza infection neutralizes the attractiveness of male odour to female mice (Mus musculus). Ethology 104, 685–694. doi: 10.1111/j.1439-0310.1998.tb00102.x
Ramalho, C. E., and Hobbs, R. J. (2012). Time for a change: dynamic urban ecology. Trends Ecol. Evol. 27, 179–188. doi: 10.1016/j.tree.2011.10.008
Rich, T. J., and Hurst, J. L. (1999). The competing countermarks hypothesis: reliable assessment of competitive ability by potential mates. Anim. Behav. 58, 1027–1037. doi: 10.1006/anbe.1999.1217
Roberts, G. (1996). Why individual vigilance declines as group size increases. Anim. Behav. 51, 1077–1086. doi: 10.1006/anbe.1996.0109
Rood, J. P. (1975). Population dynamics and food habits of the banded mongoose (Mungos mungo). Afr. J. Ecol. 13, 89–111.
Rood, J. P. (1983). Banded mongoose rescues pack member from eagle. Anim. Behav. 31, 1261–1262. doi: 10.1016/s0003-3472(83)80036-0
Roper, T., Conradt, L., Butler, J., Christian, S., Ostler, J., and Schmid, T. (1993). Territorial marking with faeces in badgers (Meles meles): a comparison of boundary and hinterland latrine use. Behaviour 127, 289–307. doi: 10.1163/156853993x00074
Wagenmakers, E.-J., and Farrell, S. (2004). AIC model selection using Akaike weights. Psychonom. Bull. Rev. 11, 192–196. doi: 10.3758/bf03206482
Washabaugh, K., and Snowdon, C. T. (1998). Chemical communication of reproductive status in female cotton-top tamarins (Saguinus oedipusoedipus). Am. J. Primatol. 45, 337–349. doi: 10.1002/(sici)1098-2345(1998)45:4<337::aid-ajp2>3.0.co;2-x
Wikenros, C., Jarnemo, A., Frisén, M., Kuijper, D. P., and Schmidt, K. (2017). Mesopredator behavioral response to olfactory signals of an apex predator. J. Ethol. 35, 161–168. doi: 10.1007/s10164-016-0504-6
Willis, C., and Poulin, R. (2000). Preference of female rats for the odours of non-parasitised males: the smell of good genes? Folia Parasitol. 47, 6–10. doi: 10.14411/fp.2000.002
Zala, S. M., Potts, W. K., and Penn, D. J. (2004). Scent-marking displays provide honest signals of health and infection. Behav. Ecol. 15, 338–344. doi: 10.1093/beheco/arh022
Keywords: scent marking, vigilance, olfactory communication, behavior, land use, pathogen transmission, heterogeneous landscapes, urbanization
Citation: Alexander KA and Nichols CA (2020) Behavior - Landscape Interactions May Create Super-Spreader Environments: Vigilance-Olfactory Interactions Across Land Type and Disease Transmission Potential in the Banded Mongoose. Front. Ecol. Evol. 8:47. doi: 10.3389/fevo.2020.00047
Received: 21 May 2019; Accepted: 14 February 2020;
Published: 12 March 2020.
Edited by:
Shannon J. McCauley, University of Toronto Mississauga, CanadaReviewed by:
Patrick Leighton, Université de Montréal, CanadaAliza le Roux, University of the Free State – Qwaqwa, South Africa
Copyright © 2020 Alexander and Nichols. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kathleen Anne Alexander, kathyalx@vt.edu
 Kathleen Anne Alexander
Kathleen Anne Alexander Carol Anne Nichols
Carol Anne Nichols