Abstract
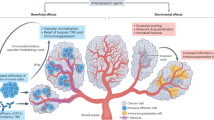
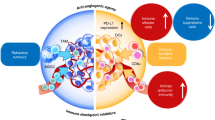
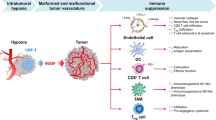
Immune checkpoint inhibitors have revolutionized medical oncology, although currently only a subset of patients has a response to such treatment. A compelling body of evidence indicates that anti-angiogenic therapy has the capacity to ameliorate antitumour immunity owing to the inhibition of various immunosuppressive features of angiogenesis. Hence, combinations of anti-angiogenic agents and immunotherapy are currently being tested in >90 clinical trials and 5 such combinations have been approved by the FDA in the past few years. In this Perspective, we describe how the angiogenesis-induced endothelial immune cell barrier hampers antitumour immunity and the role of endothelial cell anergy as the vascular counterpart of immune checkpoints. We review the antitumour immunity-promoting effects of anti-angiogenic agents and provide an update on the current clinical successes achieved when these agents are combined with immune checkpoint inhibitors. Finally, we propose that anti-angiogenic agents are immunotherapies — and vice versa — and discuss future research priorities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastaic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Wolchok, J. D. et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 377, 1345–1356 (2017).
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639 (2015).
Motzer, R. J. et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 373, 1803–1813 (2015).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Dong, H. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 (2002).
Sharma, P., Hu-Lieskovan, S., Wargo, J. A. & Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 168, 707–723 (2017).
Topalian, S. L., Taube, J. M., Anders, R. A. & Pardoll, D. M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 16, 275–287 (2016).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 541, 321–330 (2017).
Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015).
Brahmer, J. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373, 123–135 (2015).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
O’Donnell, J. S., Teng, M. W. L. & Smyth, M. J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 16, 151–167 (2019).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Vanneman, M. & Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12, 237–251 (2012).
Griffioen, A. W. & Molema, G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol. Rev. 52, 237–268 (2000).
Rahma, O. E. & Hodi, F. S. The intersection between tumor angiogenesis and immune suppression. Clin. Cancer Res. 25, 5449–5457 (2019).
Motz, G. T. & Coukos, G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat. Rev. Immunol. 11, 702–711 (2011).
Hicklin, D. J. & Ellis, L. M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 23, 1011–1027 (2005).
Gabrilovich, D. et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 2, 1096–1103 (1996).
Wada, J. et al. The contribution of vascular endothelial growth factor to the induction of regulatory T- cells in malignant effusions. Anticancer. Res. 29, 881–888 (2009).
Terme, M. et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 73, 539–549 (2013).
Huang, Y. et al. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood 110, 624–631 (2007).
Ohm, J. E. et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 101, 4878–4886 (2003).
Gavalas, N. G. et al. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br. J. Cancer 107, 1869–1875 (2012).
Voron, T. et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 212, 139–148 (2015).
Khan, K. A. & Kerbel, R. S. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat. Rev. Clin. Oncol. 15, 310–324 (2018).
Griffioen, A., Damen, C., Blijham, G. & Groenewegen, G. Tumor angiogenesis is accompanied by a decreased inflammatory response of tumor-associated endothelium. Blood 88, 667–673 (1996).
Dirkx, A. E. M. et al. Tumor angiogenesis modulates leukocyte-vessel wall interactions in Vivo by reducing endothelial adhesion molecule expression. Cancer Res. 63, 2322–2329 (2003).
Griffioen, A. W., Damen, C. A., Martinotti, S., Blijham, G. H. & Groenewegen, G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res. 56, 1111–1117 (1996).
Gajewski, T. F. et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr. Opin. Immunol. 25, 268–276 (2013).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Bindea, G. et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 39, 782–795 (2013).
Gajewski, T. F. The next hurdle in cancer immunotherapy: overcoming the non–T-cell–inflamed tumor microenvironment. Semin. Oncol. 42, 663–671 (2015).
Zhang, L. et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 348, 203–213 (2003).
Galon, J. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006).
Hegde, P. S., Karanikas, V. & Evers, S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin. Cancer Res. 22, 1865–1874 (2016).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 69, 4–10 (2005).
Carmeliet, P. & Jain, R. K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Chouaib, S., Noman, M. Z., Kosmatopoulos, K. & Curran, M. A. Hypoxic stress: obstacles and opportunities for innovative immunotherapy of cancer. Oncogene 36, 439–445 (2017).
Fukumura, D., Kloepper, J., Amoozgar, Z., Duda, D. G. & Jain, R. K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat. Rev. Clin. Oncol. 15, 325–340 (2018).
Huang, Y. et al. Improving immune–vascular crosstalk for cancer immunotherapy. Nat. Rev. Immunol. 18, 195–203 (2018).
Ramjiawan, R. R., Griffioen, A. W. & Duda, D. G. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 20, 185–204 (2017).
Socinski, M. A. et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N. Engl. J. Med. 378, 2288–2301 (2018).
Rini, B. I. et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1116–1127 (2019).
Motzer, R. J. et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380, 1103–1115 (2019).
Makker, V. et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: an interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 20, 711–718 (2019).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Chen, D. S. & Hurwitz, H. Combinations of bevacizumab with cancer immunotherapy. Cancer J. 24, 193–204 (2018).
Pober, J. S. & Sessa, W. C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 7, 803–815 (2007).
Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 15, 692–704 (2015).
von Andrian, U. H. & Mackay, C. R. T-cell function and migration — two sides of the same coin. N. Engl. J. Med. 343, 1020–1034 (2000).
Bevilacqua, M. P. Endothelial-leukcoyte adhesion molecules. Annu. Rev. Immuonol. 11, 767–804 (1993).
Springer, T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell 76, 301–314 (1994).
Klein, D. The tumor vascular endothelium as decision maker in cancer therapy. Front. Oncol. 8, 367 (2018).
Kuzu, I. et al. Heterogeneity of vascular endothelial cells with relevance to diagnosis of vascular tumours. J. Clin. Pathol. 45, 143–148 (1992).
Berger, R. et al. Expression of platelet-endothelial cell adhesion molecule-1 (PECAM-1) during melanoma-induced angiogenesis in vivo. J. Cutan. Pathol. 20, 399–406 (1993).
Dewhirst, M. W. & Dewhirst, M. W. Diminished leukocyte-endothelium interaction in tumor microvessels. Cancer Res. 52, 4265–4268 (1992).
Shibata, Y. et al. Suppressive effect of basic fibroblast growth factor on transendothelial emigration of CD4+ T-lymphocyte. Cancer Res. 54, 4729–4733 (1994).
Piali, L., Fichtel, A., Terpe, H. J., Imhof, B. A. & Gisler, R. H. Endothelial vascular cell adhesion molecule 1 expression is suppressed by melanoma and carcinoma. J. Exp. Med. 181, 811–816 (1995).
Fukumura, D. et al. Tumor necrosis factor α-induced leukocyte adhesion in normal and tumor vessels: effect of tumor type, transplantation site, and host strain. Cancer Res. 55, 4824–4829 (1995).
Schmidt, J. et al. Reduced basal and stimulated leukocyte adherence in tumor endothelium of experimental pancreatic cancer. Int. J. Gastrointest. Cancer 26, 173–180 (1999).
Tromp, S. C. et al. Tumor angiogenesis factors reduce leukocyte adhesion in vivo. Int. Immunol. 12, 671–676 (2000).
Bouma-ter Steege, J. C. et al. Angiogenic profile of breast carcinoma determines leukocyte infiltration. Clin. Cancer Res. 10, 7171–7178 (2004).
Flati, V. et al. Endothelial cell anergy is mediated by bFGF through the sustained activation of p38-MAPK and NF-κB inhibition. Int. J. Immunopathol. Pharmacol. 19, 761–773 (2006).
Hellebrekers, D. M. E. I. et al. Epigenetic regulation of tumor endothelial cell anergy: silencing of intercellular adhesion molecule-1 by histone modifications. Cancer Res. 66, 10770–10777 (2006).
Kubes, P., Suzuki, M. & Granger, D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl Acad. Sci. USA 88, 4651–4655 (1991).
De Caterina, R. et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J. Clin. Invest. 96, 60–68 (1995).
Bouzin, C., Brouet, A., De Vriese, J., DeWever, J. & Feron, O. Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J. Immunol. 178, 1505–1511 (2007).
Buckanovich, R. J. et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat. Med. 14, 28–36 (2008).
Melder, R. J. et al. During angiogenesis, vascular endothelial growth factor regulate natural killer cell adhesion to tumor endothelium. Nat. Med. 2, 992–997 (1996).
Kevil, C. G. et al. Intercellular adhesion molecule-1 (ICAM-1) regulates endothelial cell motility through a nitric oxide-dependent pathway. J. Biol. Chem. 279, 19230–19238 (2004).
Agata, Y. et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 8, 765–772 (1996).
Blackburn, S. D. et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10, 29–37 (2009).
Baitsch, L. et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360 (2011).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000).
Keir, M. E., Butte, M. J., Freeman, G. J. & Sharpe, A. H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008).
Nussbaum, C. et al. Neutrophil and endothelial adhesive function during human fetal ontogeny. J. Leukoc. Biol. 93, 175–184 (2013).
Larson, B. J., Longaker, M. T. & Lorenz, H. P. Scarless fetal wound healing: a basic science review. Plast. Reconstr. Surg. 126, 1172–1180 (2010).
Lo, D. D., Zimmermann, A. S., Nauta, A., Longaker, M. T. & Lorenz, H. P. Scarless fetal skin wound healing update. Birth Defects Res. C. Embryo Today Rev. 96, 237–247 (2012).
Reinke, J. M. & Sorg, H. Wound repair and regeneration. Eur. Surg. Res. 49, 35–43 (2012).
Demir, R., Seval, Y. & Huppertz, B. Vasculogenesis and angiogenesis in the early human placenta. Acta Histochem. 109, 257–265 (2007).
Shechter, R., London, A. & Schwartz, M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat. Rev. Immunol. 13, 206–218 (2013).
Hua, Y. & Bergers, G. Tumors vs. chronic wounds: an immune cell’s perspective. Front. Immunol. 10, 2178 (2019).
Landén, N. X., Li, D. & Ståhle, M. Transition from inflammation to proliferation: a critical step during wound healing. Cell. Mol. Life Sci. 73, 3861–3885 (2016).
De Palma, M., Biziato, D. & Petrova, T. V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 17, 457–474 (2017).
Missiaen, R., Mazzone, M. & Bergers, G. The reciprocal function and regulation of tumor vessels and immune cells offers new therapeutic opportunities in cancer. Semin. Cancer Biol. 52, 107–116 (2018).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Zou, W. & Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 8, 467–477 (2008).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Flier, J. S., Underhill, L. H. & Dvorak, H. F. Tumors: wounds that do not heal. N. Engl. J. Med. 315, 1650–1659 (1986).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Gettinger, S. N. et al. Overall survival and long-term safety of nivolumab (Anti–programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non–small-cell lung cancer. J. Clin. Oncol. 33, 2004–2012 (2015).
Topalian, S. L. et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 32, 1020–1030 (2014).
Griffioen, A. W. et al. Angiogenesis inhibitors overcome tumor induced endothelial cell anergy. Int. J. Cancer 80, 315–319 (1999).
Tabruyn, S. P. et al. The angiostatic 16K human prolactin overcomes endothelial cell anergy and promotes leukocyte infiltration via nuclear factor-κB activation. Mol. Endocrinol. 21, 1422–1429 (2007).
Zhang, H. & Issekutz, A. C. Down-modulation of monocyte transendothelial migration and endothelial adhesion molecule expression by fibroblast growth factor. Am. J. Pathol. 160, 2219–2230 (2002).
Dirkx, A. E. M. et al. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 20, 621–630 (2006).
Dings, R. P. M. et al. Enhancement of T-cell-mediated antitumor response: angiostatic adjuvant to immunotherapy against cancer. Clin. Cancer Res. 17, 3134–3145 (2011).
Thijssen, V. L., Heusschen, R., Caers, J. & Griffioen, A. W. Galectin expression in cancer diagnosis and prognosis: a systematic review. Biochim. Biophys. Acta Rev. Cancer 1855, 235–247 (2015).
Thijssen, V. L. J. L. et al. Galectin-1 is essential in tumor angiogenesis and is a target for antiangiogenesis therapy. Proc. Natl Acad. Sci. USA 103, 15975–15980 (2006).
Rabinovich, G. A. & Toscano, M. A. Turning ‘sweet’ on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 9, 338–352 (2009).
Perillo, N. L., Pace, K. E., Seilhamer, J. J. & Baum, L. G. Apoptosis of T cells mediated by galectin-1. Nature 378, 736–739 (1995).
Chung, C. D., Patel, V. P., Moran, M., Lewis, L. A. & Miceli, M. C. Galectin-1 induces partial TCR ζ-chain phosphorylation and antagonizes processive TCR signal transduction. J. Immunol. 165, 3722–3729 (2000).
Blaser, C. et al. β-Galactoside-binding protein secreted by activated T cells inhibits antigen-induced proliferation of T cells. Eur. J. Immunol. 28, 2311–2319 (1998).
Matarrese, P. et al. Galectin-1 sensitizes resting human T lymphocytes to Fas (CD95)-mediated cell death via mitochondrial hyperpolarization, budding, and fission. J. Biol. Chem. 280, 6969–6985 (2005).
Clausse, N., Van Den Brûle, F., Waltregny, D., Garnier, F. & Castronovo, V. Galectin-1 expression in prostate tumor-associated capillary endothelial cells is increased by prostate carcinoma cells and modulates heterotypic cell-cell adhesion. Angiogenesis 3, 317–325 (1999).
He, J. & Baum, L. G. Endothelial cell expression of galectin-1 induced by prostate cancer cells inhibits T-cell transendothelial migration. Lab. Invest. 86, 578–590 (2006).
Norling, L. V., Sampaio, A. L. F., Cooper, D. & Perretti, M. Inhibitory control of endothelial galectin-1 on in vitro and in vivo lymphocyte trafficking. FASEB J. 22, 682–690 (2008).
La, M. et al. A novel biological activity for galectin-1. Am. J. Pathol. 163, 1505–1515 (2003).
Rubinstein, N. et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection. Cancer Cell 5, 241–251 (2004).
Dalotto-Moreno, T. et al. Targeting galectin-1 overcomes breast cancer-associated immunosuppression and prevents metastatic disease. Cancer Res. 73, 1107–1117 (2013).
Nambiar, D. K. et al. Galectin-1–driven T cell exclusion in the tumor endothelium promotes immunotherapy resistance. J. Clin. Invest. 129, 5553–5567 (2019).
Motz, G. T. et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 20, 607–615 (2014).
Huang, X. et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J. Exp. Med. 207, 505–520 (2010).
Rodig, N. et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 33, 3117–3126 (2003).
Mazanet, M. M. & Hughes, C. C. W. B7-H1 Is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J. Immunol. 169, 3581–3588 (2002).
Grabie, N. et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell–mediated injury in the heart. Circulation 116, 2062–2071 (2007).
Chen, W.-J. et al. Human umbilical vein endothelial cells promote the inhibitory activation of CD4+ CD25+ Foxp3+ regulatory T cells via PD-L1. Atherosclerosis 244, 108–112 (2016).
Kamada, T. et al. PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc. Natl Acad. Sci. USA 116, 9999–10008 (2019).
Kumagai, S. et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 21, 1346–1358 (2020).
Liu, S. et al. anlotinib alters tumor immune microenvironment by downregulating PD-L1 expression on vascular endothelial cells. Cell Death Dis. 11, 309 (2020).
Allen, E. et al. Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl Med. 9, eaak9679 (2017).
Shigeta, K. et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology 71, 1247–1261 (2020).
Schmittnaegel, M. et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl Med. 9, eaak9670 (2017).
Riesenberg, R. et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin. Cancer Res. 13, 6993–7002 (2007).
Blaschitz, A. et al. Vascular endothelial expression of indoleamine 2,3-dioxygenase 1 forms a positive gradient towards the feto-maternal interface. PLoS ONE 6, e21774 (2011).
Shetty, S. et al. Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. J. Immunol. 186, 4147–4155 (2011).
Bergers, G. & Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 8, 592–603 (2008).
Ebos, J. M. L. & Kerbel, R. S. Antiangiogenic therapy: impact on invasion, disease progression, and metastasis. Nat. Rev. Clin. Oncol. 8, 210–221 (2011).
Bajou, K. et al. PAI-1 mediates the antiangiogenic and profibrinolytic effects of 16K prolactin. Nat. Med. 20, 741–747 (2014).
Griffioen, A. W. et al. Anginex, a designed peptide that inhibits angiogenesis. Biochem. J. 354, 233 (2001).
Kim, M. S. et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat. Med. 7, 437–443 (2001).
Hellebrekers, D. M. E. I. et al. Angiostatic activity of DNA methyltransferase inhibitors. Mol. Cancer Ther. 5, 467–475 (2006).
Huang, Y. et al. Dual-mechanism based CTLs infiltration enhancement initiated by Nano-sapper potentiates immunotherapy against immune-excluded tumors. Nat. Commun. 11, 622 (2020).
Weishaupt, C. et al. Activation of human vascular endothelium in melanoma metastases induces ICAM-1 and E-selectin expression and results in increased infiltration with effector lymphocytes. Exp. Dermatol. 28, 1258–1269 (2019).
Calcinotto, A. et al. Targeting TNF-α to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. J. Immunol. 188, 2687–2694 (2012).
Fisher, D. T. et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J. Clin. Invest. 121, 3846–3859 (2011).
Gabrilovich, D. I., Ishida, T., Nadaf, S., Ohm, J. E. & Carbone, D. P. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin. Cancer Res. 5, 2963–2970 (1999).
Ishida, T., Oyama, T., Carbone, D. P. & Gabrilovich, D. I. Defective function of langerhans cells in tumor-bearing animals is the result of defective maturation from hemopoietic progenitors. J. Immunol. 161, 4842–4851 (1998).
Manzoni, M. et al. Immunological effects of bevacizumab-based treatment in metastatic colorectal cancer. Oncology 79, 187–196 (2010).
Martino, E. et al. Immune-modulating effects of bevacizumab in metastatic non-small-cell lung cancer patients. Cell Death Discov. 2, 16025 (2016).
Ozao-Choy, J. et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 69, 2514–2522 (2009).
Finke, J. H. et al. Sunitinib reverses type-1 immune suppression and decreases T-regulatory cells in renal cell carcinoma patients. Clin. Cancer Res. 14, 6674–6682 (2008).
Adotevi, O. et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J. Immunother. 33, 991–998 (2010).
Ko, J. S. et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin. Cancer Res. 15, 2148–2157 (2009).
Jain, R. K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Carmeliet, P. & Jain, R. K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 10, 417–427 (2011).
Weiss, A. et al. Angiostatic treatment prior to chemo- or photodynamic therapy improves anti-tumor efficacy. Sci. Rep. 5, 8990 (2015).
Shrimali, R. K. et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 70, 6171–6180 (2010).
Huang, Y. et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl Acad. Sci. USA 109, 17561–17566 (2012).
Hamzah, J. et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 453, 410–414 (2008).
Mazzieri, R. et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell 19, 512–526 (2011).
Di Tacchio, M. et al. Tumor vessel normalization, immunostimulatory reprogramming, and improved survival in glioblastoma with combined inhibition of PD-1, angiopoietin-2, and VEGF. Cancer Immunol. Res. 7, 1910–1927 (2019).
Johansson-Percival, A. et al. Intratumoral LIGHT restores pericyte contractile properties and vessel integrity. Cell Rep. 13, 2687–2698 (2015).
He, B. et al. Vascular targeting of LIGHT normalizes blood vessels in primary brain cancer and induces intratumoural high endothelial venules. J. Pathol. 245, 209–221 (2018).
Johansson-Percival, A. et al. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat. Immunol. 18, 1207–1217 (2017).
Otterdal, K. et al. Platelet-derived LIGHT induces inflammatory responses in endothelial cells and monocytes. Blood 108, 928–935 (2006).
Girard, J.-P. & Springer, T. A. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol. Today 16, 449–457 (1995).
Milutinovic, S., Abe, J., Godkin, A., Stein, J. V. & Gallimore, A. The dual role of high endothelial venules in cancer progression versus immunity. Trends Cancer 7, 214–225 (2021).
Sawa, Y. et al. Immunohistochemical study on leukocyte adhesion molecules expressed on lymphatic endothelium. Microvasc. Res. 57, 292–297 (1999).
Guislain, A. et al. Sunitinib pretreatment improves tumor-infiltrating lymphocyte expansion by reduction in intratumoral content of myeloid-derived suppressor cells in human renal cell carcinoma. Cancer Immunol. Immunother. 64, 1241–1250 (2015).
Ragusa, S. et al. Antiangiogenic immunotherapy suppresses desmoplastic and chemoresistant intestinal tumors in mice. J. Clin. Invest. 130, 1199–1216 (2020).
Li, H. et al. CAIX-specific CAR-T cells and sunitinib show synergistic effects against metastatic renal cancer models. J. Immunother. 43, 16–28 (2020).
Bocca, P. et al. Bevacizumab-mediated tumor vasculature remodelling improves tumor infiltration and antitumor efficacy of GD2-CAR T cells in a human neuroblastoma preclinical model. Oncoimmunology 7, e1378843 (2018).
Manning, E. A. et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin. Cancer Res. 13, 3951–3959 (2007).
Yasuda, S. et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin. Exp. Immunol. 172, 500–506 (2013).
Leenders, W. P. J., Küsters, B. & de Waal, R. M. W. Vessel co-option: how tumors obtain blood supply in the absence of sprouting angiogenesis. Endothelium 9, 83–87 (2002).
Maniotis, A. J. et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am. J. Pathol. 155, 739–752 (1999).
Kuczynski, E. A., Vermeulen, P. B., Pezzella, F., Kerbel, R. S. & Reynolds, A. R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 16, 469–493 (2019).
Bridgeman, V. L. et al. Vessel co-option is common in human lung metastases and mediates resistance to anti-angiogenic therapy in preclinical lung metastasis models. J. Pathol. 241, 362–374 (2017).
Hu, J. et al. Gene expression signature for angiogenic and nonangiogenic non-small-cell lung cancer. Oncogene 24, 1212–1219 (2005).
Van den Eynden, G. G. et al. The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin. Exp. Metastasis 29, 541–549 (2012).
Van Dam, P. J. et al. Histopathological growth patterns as a candidate biomarker for immunomodulatory therapy. Semin. Cancer Biol. 52, 86–93 (2018).
Hendrix, M. J. C., Seftor, E. A., Hess, A. R. & Seftor, R. E. B. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat. Rev. Cancer 3, 411–421 (2003).
Paulis, Y. W. J., Soetekouw, P. M. M. B., Verheul, H. M. W., Tjan-Heijnen, V. C. G. & Griffioen, A. W. Signalling pathways in vasculogenic mimicry. Biochim. Biophys. Acta Rev. Cancer 1806, 18–28 (2010).
van der Schaft, D. W. J. et al. Tumor cell plasticity in Ewing sarcoma, an alternative circulatory system stimulated by hypoxia. Cancer Res. 65, 11520–11528 (2005).
van der Schaft, D. W. J. et al. Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. J. Natl Cancer Inst. 96, 1473–1477 (2004).
Vartanian, A. et al. Inhibitor of vasculogenic mimicry restores sensitivity of resistant melanoma cells to DNA-damaging agents. Melanoma Res. 27, 8–16 (2017).
van Beijnum, J. R., Nowak-Sliwinska, P., Huijbers, E. J. M., Thijssen, V. L. & Griffioen, A. W. The great escape; the hallmarks of resistance to antiangiogenic therapy. Pharmacol. Rev. 67, 441–461 (2015).
Makker, V. et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J. Clin. Oncol. 38, 2981–2992 (2020).
Motzer, R. J. et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat. Med. 26, 1733–1741 (2020).
McDermott, D. F. et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 24, 749–757 (2018).
Giraldo, N. A. et al. Tumor-infiltrating and peripheral blood T-cell immunophenotypes predict early relapse in localized clear cell renal cell carcinoma. Clin. Cancer Res. 23, 4416–4428 (2017).
Braun, D. A. et al. Interplay of somatic alterations and immune infiltration modulates response to PD-1 blockade in advanced clear cell renal cell carcinoma. Nat. Med. 26, 909–918 (2020).
Lan, C. et al. Camrelizumab plus apatinib in patients with advanced cervical cancer (CLAP): a multicenter, open-label, single-arm, phase II trial. J. Clin. Oncol. 38, 4095–4106 (2020).
Jaini, R., Rayman, P., Cohen, P. A., Finke, J. H. & Tuohy, V. K. Combination of sunitinib with anti-tumor vaccination inhibits T cell priming and requires careful scheduling to achieve productive immunotherapy. Int. J. Cancer 134, 1695–1705 (2014).
Liu, X.-D. et al. Resistance to antiangiogenic therapy is associated with an immunosuppressive tumor microenvironment in metastatic renal cell carcinoma. Cancer Immunol. Res. 3, 1017–1029 (2015).
Taube, J. M. et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl Med. 4, 127ra37 (2012).
Ciciola, P., Cascetta, P., Bianco, C., Formisano, L. & Bianco, R. Combining immune checkpoint inhibitors with anti-angiogenic agents. J. Clin. Med. 9, 675 (2020).
Tian, L. et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544, 250–254 (2017).
Wentink, M. Q. et al. Vaccination approach to anti-angiogenic treatment of cancer. Biochim. Biophys. Acta Rev. Cancer 1855, 155–171 (2015).
Jackson, H. J., Rafiq, S. & Brentjens, R. J. Driving CAR T-cells forward. Nat. Rev. Clin. Oncol. 13, 370–383 (2016).
Kakarla, S. & Gottschalk, S. CAR T cells for solid tumors. Cancer J. 20, 151–155 (2014).
Akbari, P., Huijbers, E. J. M., Themeli, M., Griffioen, A. W. & van Beijnum, J. R. The tumor vasculature an attractive CAR T cell target in solid tumors. Angiogenesis 22, 473–475 (2019).
Niethammer, A. G. et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat. Med. 8, 1369–1375 (2002).
Facciponte, J. G. et al. Tumor endothelial marker 1–specific DNA vaccination targets tumor vasculature. J. Clin. Invest. 124, 1497–1511 (2014).
Wentink, M. Q. et al. Targeted vaccination against the bevacizumab binding site on VEGF using 3D-structured peptides elicits efficient antitumor activity. Proc. Natl Acad. Sci. USA 113, 12532–12537 (2016).
Huijbers, E. J. M. et al. Vaccination against the extra domain-B of fibronectin as a novel tumor therapy. FASEB J. 24, 4535–4544 (2010).
Zhuang, X. et al. Robo4 vaccines induce antibodies that retard tumor growth. Angiogenesis 18, 83–95 (2015).
Huijbers, E. J. M. et al. Targeting tumor vascular CD99 inhibits tumor growth. Front. Immunol. 10, 651 (2019).
Scalia, R., Booth, G. & Lefer, D. J. Vascular endothelial growth factor attenuates leukocyte–endothelium interaction during acute endothelial dysfunction: essential role of endothelium-derived nitric oxide. FASEB J. 13, 1039–1046 (1999).
Minshall, R. D., Sessa, W. C., Stan, R. V., Anderson, R. G. W. & Malik, A. B. Caveolin regulation of endothelial function. Am. J. Physiol. Cell. Mol. Physiol. 285, L1179–L1183 (2003).
Weiss, A. et al. Rapid optimization of drug combinations for the optimal angiostatic treatment of cancer. Angiogenesis 18, 233–244 (2015).
Nowak-Sliwinska, P. et al. Optimization of drug combinations using feedback system control. Nat. Protoc. 11, 302–315 (2016).
Ho, D. Artificial intelligence in cancer therapy. Science 367, 982–983 (2020).
Sicklick, J. K. et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat. Med. 25, 744–750 (2019).
Mougel, A., Terme, M. & Tanchot, C. Therapeutic cancer vaccine and combinations with antiangiogenic therapies and immune checkpoint blockade. Front. Immunol. 10, 467 (2019).
Argentiero, A. et al. Anti-angiogenesis and immunotherapy: novel paradigms to envision tailored approaches in renal cell-carcinoma. J. Clin. Med. 9, 1594 (2020).
Galluzzi, L., Humeau, J., Buqué, A., Zitvogel, L. & Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 17, 725–741 (2020).
Formenti, S. C. et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 24, 1845–1851 (2018).
Acknowledgements
The work of P.N-S. is funded by the Swiss National Science Foundation (grant 310030_197878). The work of A.W.G. is supported by the KWF Cancer Society (grant 2018–11651).
Author information
Authors and Affiliations
Contributions
Z.R.H. and A.W.G. researched data for this article. All authors contributed to all other aspects of preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks M. de Palma, who co-reviewed with A. Martinez-Usatorre, R. Kerbel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov database: https://clinicaltrials.gov
Supplementary information
Rights and permissions
About this article
Cite this article
Huinen, Z.R., Huijbers, E.J.M., van Beijnum, J.R. et al. Anti-angiogenic agents — overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat Rev Clin Oncol 18, 527–540 (2021). https://doi.org/10.1038/s41571-021-00496-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-021-00496-y
This article is cited by
-
Self-delivery photothermal-boosted-nanobike multi-overcoming immune escape by photothermal/chemical/immune synergistic therapy against HCC
Journal of Nanobiotechnology (2024)
-
Antiangiogenic–immune-checkpoint inhibitor combinations: lessons from phase III clinical trials
Nature Reviews Clinical Oncology (2024)
-
FGFR-targeted therapeutics: clinical activity, mechanisms of resistance and new directions
Nature Reviews Clinical Oncology (2024)
-
GRIN2A mutation is a novel indicator of stratifying beneficiaries of immune checkpoint inhibitors in multiple cancers
Cancer Gene Therapy (2024)
-
Neoadjuvant nivolumab plus bevacizumab therapy improves the prognosis of triple-negative breast cancer in humanized mouse models
Breast Cancer (2024)